TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection - PubMed (original) (raw)
. 2017 Mar;10(2):493-507.
doi: 10.1038/mi.2016.60. Epub 2016 Jul 20.
J N Radin 2, R Chatuvedi 2, M B Piazuelo 2, D J Horvarth 2, H Cortado 1, Y Gu 3, B Dixon 2, C Gu 3, I Lange 4, D-Lt Koomoa 4, K T Wilson 2 5 6, H M S Algood 2 5 6, S Partida-Sánchez 1 7
Affiliations
- PMID: 27435104
- PMCID: PMC5250617
- DOI: 10.1038/mi.2016.60
TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection
S Beceiro et al. Mucosal Immunol. 2017 Mar.
Abstract
Calcium signaling in phagocytes is essential for cellular activation, migration, and the potential resolution of infection or inflammation. The generation of reactive oxygen species (ROS) via activation of NADPH (nicotinamide adenine dinucleotide phosphate)-oxidase activity in macrophages has been linked to altered intracellular calcium concentrations. Because of its role as an oxidative stress sensor in phagocytes, we investigated the function of the cation channel transient receptor potential melastatin 2 (TRPM2) in macrophages during oxidative stress responses induced by Helicobacter pylori infection. We show that Trpm2-/- mice, when chronically infected with H. pylori, exhibit increased gastric inflammation and decreased bacterial colonization compared with wild-type (WT) mice. The absence of TRPM2 triggers greater macrophage production of inflammatory mediators and promotes classically activated macrophage M1 polarization in response to H. pylori. TRPM2-deficient macrophages upon H. pylori stimulation are unable to control intracellular calcium levels, which results in calcium overloading. Furthermore, increased intracellular calcium in TRPM2-/- macrophages enhanced mitogen-activated protein kinase and NADPH-oxidase activities, compared with WT macrophages. Our data suggest that augmented production of ROS and inflammatory cytokines with TRPM2 deletion regulates oxidative stress in macrophages and consequently decreases H. pylori gastric colonization while increasing inflammation in the gastric mucosa.
Figures
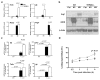
Figure 1. TRPM2 deficiency results in increased classical M1 polarization in vitro and in vivo
(a) Expression of genes associated with macrophage polarization in BMDM after 6 h of stimulation with M1-polarizing stimuli, LPS (50 ng/ml) and IFN-γ (20 ng/ml), or M2-polarizing stimuli, IL-4 (20 ng/ml) and IL-13 (20 ng/ml). Tnfa, il6, Nos2 and Arg1 expression levels were assessed by qRT-PCR and normalized to Gapdh levels. Data shown are representative of four independent experiments. (b) Arg1 and iNOS protein levels were assessed by Western blotting after 24 h of M1-and M2-polarizing stimuli treatment of BMDM cells from WT and Trpm2−/− mice. A representative blot is shown. Similar results were observed in three independent experiments. (c) BMDM from WT and Trpm2−/− mice were stimulated with H. pylori for 6 h, and RNA was isolated and subjected to qRT-PCR analysis of Il12p40, Il1b, Tnfa and Il6. Expression was normalized to GAPDH. The values shown are from four independent experiments. (d) Flow cytometry analysis of bactericidal activity. BMDM from WT and Trpm2−/− mice were incubated with H. pylori (MOI of 10) for 1 h. Cells were then washed and further incubated for 0–4 h, then lysed. The lysates were exposed to Syto 9 and propidium iodide (PI) to stain live and dead bacteria, respectively. Percentage of PI+ cells from 0.5 to 4 h after challenge is shown. (c–d) Data are representative of five independent experiments.
Figure 2. Lack of TRPM2 improves control of Helicobacter pylori infection but aggravates gastric immunopathology
(a) Gastric inflammation at 1, 3 and 6-mo after H. pylori PMSS1 infection was assessed and scored (0 to 12) on stomach tissue (corpus and antrum) in WT and T Trpm2−/− mice. Each data point represents inflammation scores from an individual animal. (b) Representative histologic images from _H. pylori_-infected WT antrum, _H. pylori_-infected WT corpus, _H. pylori_-infected TRPM2−/− antrum, _H. pylori_-infected TRPM2−/− corpus mice at 200x magnification. (c) Levels of colonization were measured by plating serial dilutions of stomach homogenates. The number of CFU was then calibrated to the weight of the tissue and log CFU/g is presented on the graphs. (d) Colonization was plotted against total inflammation for each infected mouse. The lines illustrate the best-fit linear regressions obtained for the two strains of mice with the correlation coefficient and significance as indicated. Each point represents a single mouse. Similar results were also observed in two other independent experiments. Results from experiments with 4–13 mice per group and are representative of at least three independent experiments.
Figure 3. Innate, but not adaptive, inflammatory responses are increased in _H. pylori_-infected Trpm2−/− mice
Gastric cells were isolated from H. pylori infected WT and Trpm2−/− mice sacrificed at 1-mo after challenge, and analyzed by flow cytometry for the expression of the macrophage marker F4/80. Total number of of F4/80+ (gated on CD11b+ and Ly6C+) cells per stomach is shown. (a) F4/80+ cells were isolated from the stomachs of WT and Trpm2−/− mice challenged with H. pylori, by magnet-assisted cell sorting (MACS). (b) RNA was isolated from macrophages and qRT-PCR was performed to analyze expression of Il1b, Nos2 and Arg1. Expression was normalized to Gapdh. Each point represents data from an individual animal. Results shown are from experiments with 8 to 15 mice per group. Similar results were observed in two other independent experiments. WT and Trpm2−/− mice were infected with H. pylori. At 1 and 3-mo post-infection, mice were sacrificed, gastric tissues were removed, and RNA was extracted. (c) Relative mRNA levels of Il6, Il1b, and Ifng (top panel) or Il17a, Il10, and Foxp3 (bottom panel) were measured by qRT-PCR. All qRT-PCR data were standardized to Gapdh mRNA. Relative mRNA levels of each point represent a single mouse. Results from experiments with 4 to 13 mice per group and are representative of at least three independent experiments.
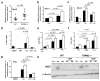
Figure 4. TRPM2 deficiency promotes NAPDH oxidase activity and oxidative stress during infection with H. pylori
(a) Nox2 mRNA levels were measured by qRT-PCR from the stomach of _H. pylori_-infected WT and Trpm2−/− mice sacrificed at 3-mo after challenge. Data were standardized to Gapdh. Each point represents a single mouse. (b) Nox2 transcript abundance in WT and TRPM2−/− BMDM was determined by qRT-PCR, and values were normalized with respect to Gapdh mRNA. (c) qRT-PCR analysis of Nox2 mRNA levels from WT and TRPM2 BMDM stimulated with H. pylori, and pretreated with DPI when indicated. Values were normalized with respect to Gapdh mRNA. (d) Release of ROS, assessed as CellROX fluorescence in WT and TRPM2−/− BMDM left unstimulated (DMSO) or stimulated with H. pylori (MOI of 50), or additional pretreatment with DPI (10 μM), presented as relative fluorescence. (e) H2O2 release measured by DHR123 oxidation in controls and _H. pylori_-stimulated WT and TRPM2−/− BMDM, presented as relative fluorescence. (f) Assessment of H2O2 release by DHR123 fluorescence in WT and TRPM2−/− BMDM left unstimulated (Control), or stimulated with PMA (1 μM) or H2O2 (10 μM), presented as relative fluorescence. (g) WT and TRPM2−/− BMDM were infected with H. pylori, with or without pretreatment with DPI (10 μM), and expression of Tnfa was examined by qRT-PCR analysis. (b–g) The values shown are from four independent experiments. (h) WT and TRPM2−/− BMDM were infected with H. pylori, with or without pretreatment with DPI (10 μM), and protein level of iNOS was examined by Western blot analysis. Data were standardized to β-actin. Western blots are representative of three independent experiments with similar results.
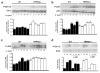
Figure 5. TRPM2 ablation augments _H. pylori_-induced MAPK activation
BMDM from WT and Trpm2−/− mice were co-cultured with H. pylori (MOI of 10) for the indicated time points, and activity of ERK (a), p38 (b) and JNK (c) were examined by Western blot analysis using phosphospecific antibodies. The total protein levels of ERK and p38 were also measured. (d) WT and TRPM2−/− BMDM were pretreated with DMSO or DPI (10 μM) before stimulation with H. pylori at the indicated times. The phosphorylation of ERK1/2 was analyzed by Western blot. (ad) Densitometric analysis for each MAPK band is displayed beneath Western blot respectively. Western blots shown are representative of three independent experiments with similar results. Statistical analyses are performed comparing differences between WT and TRPM2−/− cells at each time point (*p<0.05).
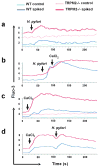
Figure 6. H. pylori modulates intracellular Ca2+ levels in macrophages
Free intracellular Ca2+ levels were measured by flow cytometry in WT and TRPM2−/− BMDM loaded with the fluorescent Ca2+ indicator Fluo-3. Cells were spiked with (a) H. pylori (MOI of 100), (b) H. pylori followed by CaCl2 (2 mM), (c) CaCl2 (2 mM), or (d) CaCl2 (2 mM) followed by H. pylori at the indicated time. Accumulation of free Ca2+ was measured by flow cytometry over the next 4 min. One of three independent experiments is shown.
Figure 7. H. pylori modulates membrane conductance of macrophages through TRPM2
(a) I-V relationship of _H. pylori_-induced membrane conductance in WT and TRPM2−/− cells. Current traces of WT (left panel) or TRPM2−/− (right panel) BMDM by voltage-clamp recording when perfused with control, (a) ADPR (200 μM) or (b) H. pylori suspension at 1×106 CFU/mL. (c) BMDM were suspended in Hank’s buffer with (circles) or without (triangles) DPI (10 μM), then electrophysiological measurements were recorded in whole-cell configuration for temporal development of inward and outward currents in WT cells by voltage-clamp recordings when cells were perfused with H. pylori containing buffer. (d) Current/voltage (IV) relationship of H. pylori alone (H. pylori), and H. pylori in WT cells preincubated with 10 μM DPI. Data are expressed as mean ± SEM, n=7–20.
Figure 8. Model of TRPM2 mediated signaling mechanisms in macrophages during H. pylori infection
(a) H. pylori engages pattern of recognition receptors (PRRs), e.g. toll-like receptors (TLRs), which recruit MyD88 and TRIF adaptor proteins that may trigger the activation of phosphatidylinositol 3-kinase (PI3K), followed by an increase of intracellular concentration of Ca2+ via release of inositol-1,4,5-trisphosphate Ins(1,4,5)P3R channel located on the endoplasmic reticulum (ER) or putative expression of lysosomal TRPM2 channel, respectively. The rise in the cytosolic Ca2+ concentration and the activity of PI3K lead to the activation of mitogen activated kinases (MAPK) and other kinases that phosphorylate the cytosolic subunits of the NADPH oxidase of phagocytes, activating their migration to the plasma membrane and enhancing production of reactive oxygen species (ROS). Increased ROS contributes to increased inflammatory gene expression through Ca2+-dependent signaling pathways, such as MAPK and NF-κB transcription factors. Oxidants, including H2O2, may mobilize ADPR from mitochondria (both H2O2 and Ca2+ can synergize with ADPR to activate TRPM2) stimulating additional cation entry across plasma membrane TRPM2. Intracellular accumulation of Na+ through TRPM2 mediates membrane depolarization, which inhibits NADPH oxidase activity, then limiting the inflammatory cascade. (b) In the absence of TRPM2, the initial PI3K dependent, IP3 mediated spike of cytosolic Ca2+ will rapidly return to baseline levels. Moreover, NADPH oxidase activity will not be restricted, resulting in further increment in ROS accumulation, and consequently, dysregulated enhancement of inflammatory signaling pathways. Persistent cell stimulation by H. pylori in the TRPM2 knockout will result in transitory Ca2+ starvation that may be the driving force for the opening of additional cation channels at the plasma membrane (e.g. TRPM7), which will enable compensatory mechanisms of Ca2+ entry favoring cytosolic Ca2+ overload, and hence, the extension of exacerbated inflammatory signals.
Similar articles
- Heme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori.
Gobert AP, Verriere T, Asim M, Barry DP, Piazuelo MB, de Sablet T, Delgado AG, Bravo LE, Correa P, Peek RM Jr, Chaturvedi R, Wilson KT. Gobert AP, et al. J Immunol. 2014 Sep 15;193(6):3013-22. doi: 10.4049/jimmunol.1401075. Epub 2014 Aug 8. J Immunol. 2014. PMID: 25108023 Free PMC article. - Matrix metalloproteinase 7 restrains Helicobacter pylori-induced gastric inflammation and premalignant lesions in the stomach by altering macrophage polarization.
Krakowiak MS, Noto JM, Piazuelo MB, Hardbower DM, Romero-Gallo J, Delgado A, Chaturvedi R, Correa P, Wilson KT, Peek RM Jr. Krakowiak MS, et al. Oncogene. 2015 Apr 2;34(14):1865-71. doi: 10.1038/onc.2014.135. Epub 2014 May 19. Oncogene. 2015. PMID: 24837365 Free PMC article. - Helicobacter pylori secretary Proteins-Induced oxidative stress and its role in NLRP3 inflammasome activation.
Kumar S, Dhiman M. Kumar S, et al. Cell Immunol. 2024 May-Jun;399-400:104811. doi: 10.1016/j.cellimm.2024.104811. Epub 2024 Feb 3. Cell Immunol. 2024. PMID: 38518686 - Transient Receptor Potential-Melastatin Channel Family Member 2: Friend or Foe.
Cheung JY, Miller BA. Cheung JY, et al. Trans Am Clin Climatol Assoc. 2017;128:308-329. Trans Am Clin Climatol Assoc. 2017. PMID: 28790515 Free PMC article. Review. - Review article: the role of inflammation in the pathogenesis of gastric cancer.
Ernst P. Ernst P. Aliment Pharmacol Ther. 1999 Mar;13 Suppl 1:13-8. doi: 10.1046/j.1365-2036.1999.00003.x. Aliment Pharmacol Ther. 1999. PMID: 10209682 Review.
Cited by
- Bidirectional regulation mechanism of TRPM2 channel: role in oxidative stress, inflammation and ischemia-reperfusion injury.
Huang P, Qu C, Rao Z, Wu D, Zhao J. Huang P, et al. Front Immunol. 2024 Jun 28;15:1391355. doi: 10.3389/fimmu.2024.1391355. eCollection 2024. Front Immunol. 2024. PMID: 39007141 Free PMC article. Review. - pH-Channeling in Cancer: How pH-Dependence of Cation Channels Shapes Cancer Pathophysiology.
Pethő Z, Najder K, Carvalho T, McMorrow R, Todesca LM, Rugi M, Bulk E, Chan A, Löwik CWGM, Reshkin SJ, Schwab A. Pethő Z, et al. Cancers (Basel). 2020 Sep 2;12(9):2484. doi: 10.3390/cancers12092484. Cancers (Basel). 2020. PMID: 32887220 Free PMC article. Review. - The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies.
Zhong T, Zhang W, Guo H, Pan X, Chen X, He Q, Yang B, Ding L. Zhong T, et al. Acta Pharm Sin B. 2022 Apr;12(4):1761-1780. doi: 10.1016/j.apsb.2021.11.001. Epub 2021 Nov 5. Acta Pharm Sin B. 2022. PMID: 35847486 Free PMC article. Review. - Functional Expression of TRPV1 Ion Channel in the Canine Peripheral Blood Mononuclear Cells.
Bujak JK, Kosmala D, Majchrzak-Kuligowska K, Bednarczyk P. Bujak JK, et al. Int J Mol Sci. 2021 Mar 20;22(6):3177. doi: 10.3390/ijms22063177. Int J Mol Sci. 2021. PMID: 33804707 Free PMC article. - TRP Channels in Stroke.
Zong P, Li CX, Feng J, Cicchetti M, Yue L. Zong P, et al. Neurosci Bull. 2024 Aug;40(8):1141-1159. doi: 10.1007/s12264-023-01151-5. Epub 2023 Nov 23. Neurosci Bull. 2024. PMID: 37995056 Review.
References
- Freedman BD. Mechanisms of calcium signaling and function in lymphocytes. Crit Rev Immunol. 2006;26:97–111. - PubMed
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS093073/NS/NINDS NIH HHS/United States
- R01 DK053620/DK/NIDDK NIH HHS/United States
- P01 CA028842/CA/NCI NIH HHS/United States
- K01 AT007324/AT/NCCIH NIH HHS/United States
- P20 GM103466/GM/NIGMS NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- I01 BX000915/BX/BLRD VA/United States
- R01 NS062720/NS/NINDS NIH HHS/United States
- I01 BX001453/BX/BLRD VA/United States
- K01 CA154758/CA/NCI NIH HHS/United States
- R01 AI092117/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials