Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial - PubMed (original) (raw)
Clinical Trial
. 2016 Sep;17(9):1283-94.
doi: 10.1016/S1470-2045(16)30167-X. Epub 2016 Jul 20.
Armando Santoro 2, Margaret Shipp 3, Pier Luigi Zinzani [ 4](#full-view-affiliation-4 "Institute of Hematology "Le A Seràgnoli", University of Bologna, Bologna, Italy."), John M Timmerman 5, Stephen Ansell 6, Philippe Armand 3, Michelle Fanale 7, Voravit Ratanatharathorn 8, John Kuruvilla 9, Jonathon B Cohen 10, Graham Collins 11, Kerry J Savage 12, Marek Trneny 13, Kazunobu Kato 14, Benedetto Farsaci 14, Susan M Parker 14, Scott Rodig 15, Margaretha G M Roemer 3, Azra H Ligon 15, Andreas Engert 16
Affiliations
- PMID: 27451390
- PMCID: PMC5541855
- DOI: 10.1016/S1470-2045(16)30167-X
Clinical Trial
Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial
Anas Younes et al. Lancet Oncol. 2016 Sep.
Abstract
Background: Malignant cells of classical Hodgkin's lymphoma are characterised by genetic alterations at the 9p24.1 locus, leading to overexpression of PD-1 ligands and evasion of immune surveillance. In a phase 1b study, nivolumab, a PD-1-blocking antibody, produced a high response in patients with relapsed and refractory classical Hodgkin's lymphoma, with an acceptable safety profile. We aimed to assess the clinical benefit and safety of nivolumab monotherapy in patients with classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin.
Methods: In this ongoing, single-arm phase 2 study, adult patients (aged ≥18 years) with recurrent classical Hodgkin's lymphoma who had failed to respond to autologous stem-cell transplantation and had either relapsed after or failed to respond to brentuximab vedotin, and with an Eastern Cooperative Oncology Group performance status score of 0 or 1, were enrolled from 34 hospitals and academic centres across Europe and North America. Patients were given nivolumab intravenously over 60 min at 3 mg/kg every 2 weeks until progression, death, unacceptable toxicity, or withdrawal from study. The primary endpoint was objective response following a prespecified minimum follow-up period of 6 months, assessed by an independent radiological review committee (IRRC). All patients who received at least one dose of nivolumab were included in the primary and safety analyses. This trial is registered with ClinicalTrials.gov, number NCT02181738.
Findings: Among 80 treated patients recruited between Aug 26, 2014, and Feb 20, 2015, the median number of previous therapies was four (IQR 4-7). At a median follow-up of 8·9 months (IQR 7·8-9·9), 53 (66·3%, 95% CI 54·8-76·4) of 80 patients achieved an IRRC-assessed objective response. The most common drug-related adverse events (those that occurred in ≥15% of patients) included fatigue (20 [25%] patients), infusion-related reaction (16 [20%]), and rash (13 [16%]). The most common drug-related grade 3 or 4 adverse events were neutropenia (four [5%] patients) and increased lipase concentrations (four [5%]). The most common serious adverse event (any grade) was pyrexia (three [4%] patients). Three patients died during the study; none of these deaths were judged to be treatment related.
Interpretation: Nivolumab resulted in frequent responses with an acceptable safety profile in patients with classical Hodgkin's lymphoma who progressed after autologous stem-cell transplantation and brentuximab vedotin. Therefore, nivolumab might be a new treatment option for a patient population with a high unmet need. Ongoing follow-up will help to assess the durability of response.
Funding: Bristol-Myers Squibb.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
AS, PLZ, GC, MR, and AHL declare no competing interests.
Figures
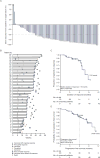
Figure 1. Efficacy outcomes per IRRC assessment
IRRC=independent radiologic review committee. Shown are the results for: Panel A – IRRC assessment of best change from baseline in target lesion for all response-evaluable patients, where crosses denote responders and the square symbol represents percentage change truncated to 100% (response evaluable was defined as patients with a best overall response of complete or partial remission, stable disease, or disease progression of target lesion[s] assessed at baseline, and at least one on-study time point with all baseline target lesion[s] assessed; negative or positive value indicates maximum tumour reduction or minimum tumour increase); Panel B – response characteristics in all responders; Panel C – duration of response; and Panel D – progression-free survival (Panel D).
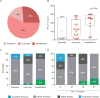
Figure 2. PD-L1/PD-L2 alterations and PD-1 ligand expression in tumour biopsies from trial patients
Panel A: the proportion of evaluable cases with polysomy, copy gain, and amplification. Panel B: box plots showing the distribution of PD-L1 H-scores across cases with polysomy, copy gain, and amplification. There is an increase in median PD-L1 H-score with increasing 9p24·1 genetic alteration (p=0·034, Kruskal-Wallis test). Panel C: objective responses among patients with Reed-Sternberg cells exhibiting polysomy, copy gain, or amplification. Panel D: objective responses by PD-L1 H-score.
Comment in
- Is nivolumab an option for Hodgkin's lymphoma?
Gisselbrecht C. Gisselbrecht C. Lancet Oncol. 2016 Sep;17(9):1183-4. doi: 10.1016/S1470-2045(16)30220-0. Epub 2016 Jul 20. Lancet Oncol. 2016. PMID: 27451389 No abstract available.
Similar articles
- Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial.
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Gianni AM, Carella A, Osmanov D, Bachanova V, Sweetenham J, Sureda A, Huebner D, Sievers EL, Chi A, Larsen EK, Hunder NN, Walewski J; AETHERA Study Group. Moskowitz CH, et al. Lancet. 2015 May 9;385(9980):1853-62. doi: 10.1016/S0140-6736(15)60165-9. Epub 2015 Mar 19. Lancet. 2015. PMID: 25796459 Clinical Trial. - Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial.
O'Connor OA, Lue JK, Sawas A, Amengual JE, Deng C, Kalac M, Falchi L, Marchi E, Turenne I, Lichtenstein R, Rojas C, Francescone M, Schwartz L, Cheng B, Savage KJ, Villa D, Crump M, Prica A, Kukreti V, Cremers S, Connors JM, Kuruvilla J. O'Connor OA, et al. Lancet Oncol. 2018 Feb;19(2):257-266. doi: 10.1016/S1470-2045(17)30912-9. Epub 2017 Dec 21. Lancet Oncol. 2018. PMID: 29276022 Free PMC article. Clinical Trial. - Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study.
Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, Fogliatto LM, Goncalves I, de Oliveira JSR, Buccheri V, Perini GF, Goldschmidt N, Kriachok I, Dickinson M, Komarnicki M, McDonald A, Ozcan M, Sekiguchi N, Zhu Y, Nahar A, Marinello P, Zinzani PL; KEYNOTE-204 investigators. Kuruvilla J, et al. Lancet Oncol. 2021 Apr;22(4):512-524. doi: 10.1016/S1470-2045(21)00005-X. Epub 2021 Mar 12. Lancet Oncol. 2021. PMID: 33721562 Clinical Trial. - Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).
Goldkuhle M, Dimaki M, Gartlehner G, Monsef I, Dahm P, Glossmann JP, Engert A, von Tresckow B, Skoetz N. Goldkuhle M, et al. Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article. Review. - Brentuximab vedotin: its role in the treatment of anaplastic large cell and Hodgkin's lymphoma.
Haddley K. Haddley K. Drugs Today (Barc). 2012 Apr;48(4):259-70. doi: 10.1358/dot.2012.48.4.1788435. Drugs Today (Barc). 2012. PMID: 22536568 Review.
Cited by
- Duration of immunotherapy in patients with advanced lung adenocarcinoma with negative driver genes: case report and literature review.
Shan Q, Wang H, Han X, Guo J, Wang Z. Shan Q, et al. Thorac Cancer. 2020 Oct;11(10):3001-3006. doi: 10.1111/1759-7714.13600. Epub 2020 Aug 24. Thorac Cancer. 2020. PMID: 32833320 Free PMC article. Review. - Necessity of neutrophil-to-lymphocyte ratio monitoring for hypothyroidism using nivolumab in patients with cancer.
Gannichida A, Nakazawa Y, Kageyama A, Utsumi H, Kuwano K, Kawakubo T. Gannichida A, et al. World J Clin Oncol. 2022 Jul 24;13(7):641-651. doi: 10.5306/wjco.v13.i7.641. World J Clin Oncol. 2022. PMID: 36157155 Free PMC article. - Uncoupling Therapeutic Efficacy from Immune-Related Adverse Events in Immune Checkpoint Blockade.
Hu W, Wang G, Wang Y, Riese MJ, You M. Hu W, et al. iScience. 2020 Sep 20;23(10):101580. doi: 10.1016/j.isci.2020.101580. eCollection 2020 Oct 23. iScience. 2020. PMID: 33083746 Free PMC article. Review. - Targeting the VEGF Pathway in Osteosarcoma.
Assi T, Watson S, Samra B, Rassy E, Le Cesne A, Italiano A, Mir O. Assi T, et al. Cells. 2021 May 18;10(5):1240. doi: 10.3390/cells10051240. Cells. 2021. PMID: 34069999 Free PMC article. Review. - Real-world retrospective analysis of immune checkpoint inhibitor therapy for relapsed or refractory Hodgkin's lymphoma.
Oyake T, Maeta T, Takahata T, Tamai Y, Kameoka Y, Takahashi N, Miyairi Y, Murai K, Shimosegawa K, Yoshida K, Inokura K, Fukuhara N, Harigae H, Sato R, Ishizawa K, Tajima K, Saitou S, Fukatsu M, Ikezoe T, Tsunoda S, Mita M, Mori J, Kowata S, Ito S. Oyake T, et al. J Clin Exp Hematop. 2024 Sep 28;64(3):191-202. doi: 10.3960/jslrt.24021. Epub 2024 Jul 31. J Clin Exp Hematop. 2024. PMID: 39085129 Free PMC article.
References
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. - PubMed
- Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24:2430–4. - PubMed
- Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical