The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release - PubMed (original) (raw)
Review
The Role of Phlebovirus Glycoproteins in Viral Entry, Assembly and Release
Martin Spiegel et al. Viruses. 2016.
Abstract
Bunyaviruses are enveloped viruses with a tripartite RNA genome that can pose a serious threat to animal and human health. Members of the Phlebovirus genus of the family Bunyaviridae are transmitted by mosquitos and ticks to humans and include highly pathogenic agents like Rift Valley fever virus (RVFV) and severe fever with thrombocytopenia syndrome virus (SFTSV) as well as viruses that do not cause disease in humans, like Uukuniemi virus (UUKV). Phleboviruses and other bunyaviruses use their envelope proteins, Gn and Gc, for entry into target cells and for assembly of progeny particles in infected cells. Thus, binding of Gn and Gc to cell surface factors promotes viral attachment and uptake into cells and exposure to endosomal low pH induces Gc-driven fusion of the viral and the vesicle membranes. Moreover, Gn and Gc facilitate virion incorporation of the viral genome via their intracellular domains and Gn and Gc interactions allow the formation of a highly ordered glycoprotein lattice on the virion surface. Studies conducted in the last decade provided important insights into the configuration of phlebovirus Gn and Gc proteins in the viral membrane, the cellular factors used by phleboviruses for entry and the mechanisms employed by phlebovirus Gc proteins for membrane fusion. Here, we will review our knowledge on the glycoprotein biogenesis and the role of Gn and Gc proteins in the phlebovirus replication cycle.
Keywords: Bunyaviridae; assembly; entry; glycoproteins; membrane fusion; phlebovirus; signal peptidase; virus attachment.
Figures
Figure 1
Replication cycle of phleboviruses. (A) Cellular attachment of phleboviruses is driven by glycoprotein interactions with host cell factors such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), heparan sulfate (HS), or non-muscle myosin heavy chain IIA (NMMHC-IIA). The binding to DC-SIGN and so far unknown entry factors induces uptake via caveolin-1-mediated endocytosis (CavME) (as for Rift Valley fever virus, RVFV) or incompletely defined clathrin-independent endocytic (CIE) mechanisms (as for Uukuniemi virus, UUKV). Ribonuclease kappa (RNaseK) promotes the internalization of virions by a yet unknown mechanism; (B) In late endosomes, the low pH induces the membrane fusion activity of the Gc protein. Expression of vesicle-associated membrane protein 3 (VAMP3) promotes UUKV penetration, while interferon-induced transmembrane protein (IFITM) 2 and IFITM3 inhibit the fusion of RVFV in late endosomes; (C) The fusion of viral and endosomal membranes allows release of the viral ribonucleoprotein complexes into the cytoplasm, the site of viral transcription and replication; (D) The viral glycoproteins Gn and Gc are translated at the rough endoplasmic reticulum (ER) as a precursor protein, Gn/Gc, which is cleaved by signal peptidase. The viral nucleoprotein and the viral polymerase are synthesized in the cytoplasm where they form together with newly produced genomic RNA (gRNA) ribonucleoprotein (RNP) complexes; (E) Binding immunoglobulin protein (BiP) and calnexin, two ER chaperones, are required for appropriate folding of Gn and Gc. Similarly, protein-disulfide-isomerase catalyzes Gn and Gc folding by promoting the formation of disulfide bonds, while calreticulin prevents misfolded Gn and Gc from being exported from the ER to the Golgi; (F) Correctly folded Gn/Gc heterodimers are transported into the Golgi apparatus where they associate with RNPs via the cytoplasmic tails of Gn during the budding process; (G) After budding of new virus particles into the Golgi is complete, virus-containing vesicles are transported to the plasma membrane where the virions are released by exocytosis. DC: dendritic cell; MФ: macrophage; CME: clathrin-mediated endocytosis; PDI: protein disulfide isomerase; CNX: calnexin.
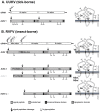
Figure 2
Coding and expression strategy of phlebovirus M-segments. Shown are the M-segments in antigenomic orientation (cRNA), the precursor glycoproteins and the membrane topology of the mature (glyco-) proteins. The antigenomic M-segment RNA serves as a template for viral transcription which results in a single mRNA. (A) UUKV as an example for tick-borne phleboviruses. The M-segment of tick-borne phleboviruses encodes only the two glycoproteins Gn and Gc. Translation of the mRNA yields one product, the Gn/Gc precursor. The precursor contains an N-terminal signal sequence preceding Gn and an internal signal sequence preceding Gc. Cleavage by the ER-associated signal peptidase complex yields Gn and Gc. Both Gn and Gc are glycosylated at _N_-glycosylation sites; (B) RVFV as an example for insect-borne phleboviruses. The M-segment of insect-borne phleboviruses encodes the non-structural protein NSm followed by the glycoproteins Gn and Gc. In case of RVFV translation initiation at different AUGs results in the expression of a nested set of polyproteins. Translation initiation at AUG 2 yields the NSm-Gn/Gc precursor protein. The precursor contains two internal signal sequences preceding Gn and Gc respectively. Cleavage by signal peptidase yields NSm, Gn and Gc. The Gn signal peptide acts as membrane anchor for NSm. Due to its membrane topology NSm is not glycosylated although it contains a potential _N_-glycosylation site. Translation at AUG 3 results in the expression of an N-terminal truncated NSm protein (NSm’) which is functionally equivalent to full-length NSm. Translation at AUG 1 yields the P78-Gc precursor protein. Signal peptidase cleaves the pre-protein after the signal sequences preceding NSm and Gc but not after the signal sequence preceding Gn which might act as membrane anchor instead. P78 is glycosylated at the _N_-glycosylation sites in the NSm and the Gn region. Note the different membrane topology of the NSm region in P78 (translation initiation at AUG 1) compared to NSm or NSm’ (translation at AUG 2 or AUG 3). Although P78 and Gc interact with each other, Gc might be unstable in the absence of Gn and therefore might be degraded in the ER. Translation at AUG 4 or 5 yields the Gn/Gc pre-protein. Signal peptidase cleaves the pre-protein after the signal sequences preceding Gn and Gc. Both Gn and Gc are _N_-glycosylated. The in vivo relevance of translation initiation at AUG 3 and 5 is not clear.
Similar articles
- Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope.
Wu Y, Zhu Y, Gao F, Jiao Y, Oladejo BO, Chai Y, Bi Y, Lu S, Dong M, Zhang C, Huang G, Wong G, Li N, Zhang Y, Li Y, Feng WH, Shi Y, Liang M, Zhang R, Qi J, Gao GF. Wu Y, et al. Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7564-E7573. doi: 10.1073/pnas.1705176114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827346 Free PMC article. - Evidence that Processing of the Severe Fever with Thrombocytopenia Syndrome Virus Gn/Gc Polyprotein Is Critical for Viral Infectivity and Requires an Internal Gc Signal Peptide.
Plegge T, Hofmann-Winkler H, Spiegel M, Pöhlmann S. Plegge T, et al. PLoS One. 2016 Nov 17;11(11):e0166013. doi: 10.1371/journal.pone.0166013. eCollection 2016. PLoS One. 2016. PMID: 27855227 Free PMC article. - Characterization of the Molecular Interactions That Govern the Packaging of Viral RNA Segments into Rift Valley Fever Phlebovirus Particles.
Tercero B, Narayanan K, Terasaki K, Makino S. Tercero B, et al. J Virol. 2021 Jun 24;95(14):e0042921. doi: 10.1128/JVI.00429-21. Epub 2021 Jun 24. J Virol. 2021. PMID: 33952635 Free PMC article. - Hantavirus Gn and Gc envelope glycoproteins: key structural units for virus cell entry and virus assembly.
Cifuentes-Muñoz N, Salazar-Quiroz N, Tischler ND. Cifuentes-Muñoz N, et al. Viruses. 2014 Apr 21;6(4):1801-22. doi: 10.3390/v6041801. Viruses. 2014. PMID: 24755564 Free PMC article. Review. - RNA Encapsidation and Packaging in the Phleboviruses.
Hornak KE, Lanchy JM, Lodmell JS. Hornak KE, et al. Viruses. 2016 Jul 15;8(7):194. doi: 10.3390/v8070194. Viruses. 2016. PMID: 27428993 Free PMC article. Review.
Cited by
- Molecular docking and simulation investigation: effect of beta-sesquiphellandrene with ionic integration on SARS-CoV2 and SFTS viruses.
Joshi A, Sunil Krishnan G, Kaushik V. Joshi A, et al. J Genet Eng Biotechnol. 2020 Nov 27;18(1):78. doi: 10.1186/s43141-020-00095-x. J Genet Eng Biotechnol. 2020. PMID: 33245459 Free PMC article. - Interaction between the SFTSV envelope glycoprotein Gn and STING inhibits the formation of the STING-TBK1 complex and suppresses the NF-κB signaling pathway.
Jia Y, Li F, Liu Z, Liu S, Huang M, Gao X, Su X, Wang Z, Wang T. Jia Y, et al. J Virol. 2024 Mar 19;98(3):e0181523. doi: 10.1128/jvi.01815-23. Epub 2024 Feb 29. J Virol. 2024. PMID: 38421179 Free PMC article. - Immune escape mechanisms of severe fever with thrombocytopenia syndrome virus.
Wang T, Xu L, Zhu B, Wang J, Zheng X. Wang T, et al. Front Immunol. 2022 Jul 28;13:937684. doi: 10.3389/fimmu.2022.937684. eCollection 2022. Front Immunol. 2022. PMID: 35967309 Free PMC article. Review. - Immune Modulation and Immune-Mediated Pathogenesis of Emerging Tickborne Banyangviruses.
Mendoza CA, Ebihara H, Yamaoka S. Mendoza CA, et al. Vaccines (Basel). 2019 Sep 20;7(4):125. doi: 10.3390/vaccines7040125. Vaccines (Basel). 2019. PMID: 31547199 Free PMC article. Review. - Potent neutralization of Rift Valley fever virus by human monoclonal antibodies through fusion inhibition.
Chapman NS, Zhao H, Kose N, Westover JB, Kalveram B, Bombardi R, Rodriguez J, Sutton R, Genualdi J, LaBeaud AD, Mutuku FM, Pittman PR, Freiberg AN, Gowen BB, Fremont DH, Crowe JE Jr. Chapman NS, et al. Proc Natl Acad Sci U S A. 2021 Apr 6;118(14):e2025642118. doi: 10.1073/pnas.2025642118. Proc Natl Acad Sci U S A. 2021. PMID: 33782133 Free PMC article.
References
- Plyusnin A., Beaty B.J., Elliott R.M., Goldbach R., Kormelink R., Lundkvist Å., Schmaljohn C.S., Tesh R.B. Bunyaviridae. In: King A.M.Q., Lefkowitz E., Adams M.J., Carstens E.B., editors. Virus Taxonomy. Classification and Nomenclature of Viruses. Ninth Report of the International Committee on Taxonomy of Viruses. Academic Press; London/Waltham, UK: San Diego, CA, USA: 2012. pp. 724–741.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous