Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture - PubMed (original) (raw)
. 2017 Oct;66(10):1802-1810.
doi: 10.1136/gutjnl-2016-311447. Epub 2016 Jul 25.
Fanny Grillet 1 2 3, Elsa Bayet 1 2 3, Olivia Villeronce 1 2 3, Ebba Louise Lagerqvist 1 2 3, Sebastian Lunke 4, Emmanuelle Charafe-Jauffret 5, Kym Pham 4 6, Christina Molck 4, Nathalie Rolland 7, Jean François Bourgaux 8, Michel Prudhomme 9, Claire Philippe 9, Sophie Bravo 10, Jean Christophe Boyer 10, Lucile Canterel-Thouennon 11, Graham Roy Taylor 4, Arthur Hsu 4, Jean Marc Pascussi 1 2 3, Frédéric Hollande 1 2 3 4, Julie Pannequin 1 2 3
Affiliations
- PMID: 27456153
- PMCID: PMC5595103
- DOI: 10.1136/gutjnl-2016-311447
Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture
Fanny Grillet et al. Gut. 2017 Oct.
Abstract
Objective: Although counting of circulating tumour cells (CTC) has attracted a broad interest as potential markers of tumour progression and treatment response, the lack of functional characterisation of these cells had become a bottleneck in taking these observations to the clinic. Our objective was to culture these cells in order to understand them and exploit their therapeutic potential to the full.
Design: Here, hypothesising that some CTC potentially have cancer stem cell (CSC) phenotype, we generated several CTC lines from the blood of patients with advanced metastatic colorectal cancer (CRC) based on their self-renewal abilities. Multiple standard tests were then employed to characterise these cells.
Results: Our CTC lines self-renew, express CSC markers and have multilineage differentiation ability, both in vitro and in vivo. Patient-derived CTC lines are tumorigenic in subcutaneous xenografts and are also able to colonise the liver after intrasplenic injection. RNA sequencing analyses strikingly demonstrate that drug metabolising pathways represent the most upregulated feature among CTC lines in comparison with primary CRC cells grown under similar conditions. This result is corroborated by the high resistance of the CTC lines to conventional cytotoxic compounds.
Conclusions: Taken together, our results directly demonstrate the existence of patient-derived colorectal CTCs that bear all the functional attributes of CSCs. The CTC culture model described here is simple and takes <1 month from blood collection to drug testing, therefore, routine clinical application could facilitate access to personalised medicine.
Clinical trial registration: ClinicalTrial.gov NCT01577511.
Keywords: COLORECTAL CANCER; DRUG TOXICITY; LIVER METASTASES.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures
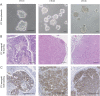
Figure 1
(A) Images of spheroids formed by circulating tumour cell (CTC)41, CTC44 and CTC45 lines (scale bar 50 μm). (B) H&E staining on tumours following subcutaneous injections of CTC lines into nude mice (scale bar 250 µm). (C) CK20 staining on tumours following subcutaneous injections of CTC lines.
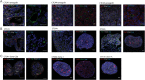
Figure 2
(A) Immunofluorescent staining of tumour xenografts obtained after subcutaneous injection of circulating tumour cell (CTC) lines into the flank of nude mice (scale bar 20 μm). (B) Immunofluorescent staining of tumour spheres formed in vitro from CTC lines (scale bar 20 μm). (C) Immunofluorescent staining of representative tumour spheres derived from single-cell clones of CTC lines (scale bar 20 μm). Names of stained intestinal and epithelial markers are specified within each photograph in the corresponding colour. E-cadherin (ECad) and cytokeratin 20 (CK20) are epithelial markers. Mucin 2 (Muc2) stains goblet cells, villin stains enterocytes and chromogranin A (CgA) stains enteroendocrine cells.
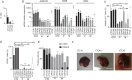
Figure 3
(A). Cancer stem cell (CSC) frequency quantified in circulating tumour cell (CTC) lines after more than seven passages as tumour spheres using the extreme dilution assay (ELDA). Presence or absence of spheres is quantified as a binary outcome and stem cell frequency is calculated, and expressed as percentage of total cell number. (B) Expression of mRNAs encoding CSC markers such as ALDH1A1, CD26 and CD44, measured using reverse transcription-quantitative PCR analysis in CTC lines and cells derived from primary colon tumours (P) or liver metastases (M) of patients with CRC. Expression of mRNAs is expressed relative to the mean expression level across all primary and metastatic tumour-derived cell lines (which was set to 1). Results are expressed as mean±SEM, n>3, statistical analyses were performed using a Mann–Whitney U test. (C) Percentage of cells with high ALDH-activity in CTC lines, HT29 and tumour-derived cell lines, quantified using the Aldefluor assay kit (STEMCELL Technologies) and measured by flow cytometry. (D) Percentage of CD26-positive cells in CTC lines, HT29 and tumour-derived cell lines quantified by flow cytometry. (C and D) Results are expressed as mean±SEM, n>3, statistical analysis: Mann–Whitney U test comparing the mean value of each group of cells lines (CTC, P and M). (E) Percentage of CD44-positive (grey bars) and CD44 v6-positive (black bars) cells in CTC, HT29 and tumour-derived cell lines analysed by flow cytometry. (F) Photographs of liver metastases formed after intrasplenic injection of CTC lines in NOD/SCID mice (scale bar 1 cm).
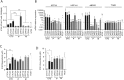
Figure 4
(A) IC50 of 5-FU + SN-38 (active metabolite of irinotecan), a common combination of chemotherapies, on the cell viability of circulating tumour cell (CTC) lines, primary (P) or metastatic (M) tumour-derived cell lines and HT29, quantified using the Cell Titer Glow assay. Results are expressed as mean±SEM, n>3, statistical analysis: Mann–Whitney U test comparing the mean value of each group of cells lines (CTC, P and M). (B) Relative expression of mRNAs encoding proteins involved in chemotherapy resistance (UGT1A, UGT1A1, MDR1, ABCG2 and TYMS) quantified by qPCR on CTC lines, P or M tumour-derived cell lines. Expression of mRNAs is expressed relative to the mean expression level across all primary and metastatic tumour-derived cell lines, which was set to 1. Results are expressed as mean±SEM, n>3, statistical analysis was performed by Mann–Whitney U test. (C) IC50 of regorafenib (multikinase inhibitor) on the cell viability of CTC lines, P or M tumour-derived cell lines and HT29, quantified using the Cell Titer Glow assay. (D) IC50 of vemurafenib (BRAF inhibitor) on the cell viability of CTC lines, P or M tumour-derived cell lines and HT29. Results are expressed as mean±SEM with n>3. Statistical analysis: Mann–Whitney U test comparing mean of each cell lines subgroup (A) or each cell line (B and C).
Similar articles
- Circulating tumor cells exhibit stem cell characteristics in an orthotopic mouse model of colorectal cancer.
Schölch S, García SA, Iwata N, Niemietz T, Betzler AM, Nanduri LK, Bork U, Kahlert C, Thepkaysone ML, Swiersy A, Büchler MW, Reissfelder C, Weitz J, Rahbari NN. Schölch S, et al. Oncotarget. 2016 May 10;7(19):27232-42. doi: 10.18632/oncotarget.8373. Oncotarget. 2016. PMID: 27029058 Free PMC article. - Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice.
Wang D, Fu L, Sun H, Guo L, DuBois RN. Wang D, et al. Gastroenterology. 2015 Dec;149(7):1884-1895.e4. doi: 10.1053/j.gastro.2015.07.064. Epub 2015 Aug 7. Gastroenterology. 2015. PMID: 26261008 Free PMC article. - Colorectal Cancer Stem Cells Acquire Chemoresistance Through the Upregulation of F-Box/WD Repeat-Containing Protein 7 and the Consequent Degradation of c-Myc.
Izumi D, Ishimoto T, Miyake K, Eto T, Arima K, Kiyozumi Y, Uchihara T, Kurashige J, Iwatsuki M, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Watanabe M, Goel A, Tan P, Baba H. Izumi D, et al. Stem Cells. 2017 Sep;35(9):2027-2036. doi: 10.1002/stem.2668. Epub 2017 Jul 31. Stem Cells. 2017. PMID: 28699179 - Non-coding RNAs Functioning in Colorectal Cancer Stem Cells.
Fanale D, Barraco N, Listì A, Bazan V, Russo A. Fanale D, et al. Adv Exp Med Biol. 2016;937:93-108. doi: 10.1007/978-3-319-42059-2_5. Adv Exp Med Biol. 2016. PMID: 27573896 Review. - Cancer stem cells and their implication in breast cancer.
Carrasco E, Alvarez PJ, Prados J, Melguizo C, Rama AR, Aránega A, Rodríguez-Serrano F. Carrasco E, et al. Eur J Clin Invest. 2014 Jul;44(7):678-87. doi: 10.1111/eci.12276. Epub 2014 May 22. Eur J Clin Invest. 2014. PMID: 24766664 Review.
Cited by
- Combined preoperative prognostic nutritional index and D-dimer score predicts outcome in colorectal cancer.
Zhu S, Yin J, Ye Q, Xiang J, Zhang Z, Yan B. Zhu S, et al. BMC Surg. 2023 Feb 7;23(1):30. doi: 10.1186/s12893-023-01925-8. BMC Surg. 2023. PMID: 36750842 Free PMC article. - CD44v3 is a marker of invasive cancer stem cells driving metastasis in gastric carcinoma.
Giraud J, Seeneevassen L, Rousseau B, Bouriez D, Sifré E, Giese A, Nguyen TL, Tiffon C, Lippi Y, Azzi-Martin L, Pannequin J, Ménard A, Bessède E, Staedel C, Mégraud F, Belleannée G, Lehours P, Gronnier C, Dubus P, Varon C. Giraud J, et al. Gastric Cancer. 2023 Mar;26(2):234-249. doi: 10.1007/s10120-022-01357-y. Epub 2022 Dec 18. Gastric Cancer. 2023. PMID: 36528833 Free PMC article. - Longitudinal Monitoring of Intra-Tumoural Heterogeneity Using Optical Barcoding of Patient-Derived Colorectal Tumour Models.
Shembrey C, Smith J, Grandin M, Williams N, Cho HJ, Mølck C, Behrenbruch C, Thomson BN, Heriot AG, Merino D, Hollande F. Shembrey C, et al. Cancers (Basel). 2022 Jan 24;14(3):581. doi: 10.3390/cancers14030581. Cancers (Basel). 2022. PMID: 35158849 Free PMC article. - Postoperative serum interleukin-6 levels correlate with survival in stage I-III colorectal cancer.
Feng S, Li Z, Liu M, Ye Q, Xue T, Yan B. Feng S, et al. BMC Gastroenterol. 2023 May 16;23(1):156. doi: 10.1186/s12876-023-02800-9. BMC Gastroenterol. 2023. PMID: 37194025 Free PMC article. - Clinical application of liquid biopsy in colorectal cancer: detection, prediction, and treatment monitoring.
Tao XY, Li QQ, Zeng Y. Tao XY, et al. Mol Cancer. 2024 Jul 16;23(1):145. doi: 10.1186/s12943-024-02063-2. Mol Cancer. 2024. PMID: 39014366 Free PMC article. Review.
References
- Dobrila-Dintinjana R, Vanis N, Dintinjana M, et al. . Etiology and oncogenesis of pancreatic carcinoma. Coll Antropol 2012;36:1063–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous