ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2 - PubMed (original) (raw)
Randomized Controlled Trial
ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2
Renee' H Martin et al. Stroke. 2016 Sep.
Abstract
Background and purpose: The ALIAS (Albumin in Acute Ischemic Stroke) part 1 and 2 trials evaluated whether 25% human serum albumin improves clinical outcomes after acute ischemic stroke above and beyond standard of care using similar protocols. The part 1 trial ended prematurely because of safety concerns, and the part 2 trial terminated early because of futility of finding a statistically significant effect of albumin over saline (control) administration. We combine the subject-level data of the part 1 and 2 trials to reevaluate the efficacy and safety outcomes with the larger sample size.
Methods: The combined data analyses closely follow those conducted in the part 2 trial. The primary outcome is the composite of the modified Rankin Scale and the National Institutes of Health Stroke Scale defined as a composite of modified Rankin Scale score 0 to 1 and National Institutes of Health Stroke Scale score 0 to 1 at 90 days from randomization. The unadjusted analyses use a simple Chi-square test, and those adjusting for baseline covariates use a generalized linear model with log link (to obtain relative risks).
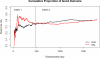
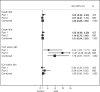
Results: The participant characteristics at baseline were generally similar between the treatment groups and between the trials; however, thrombolysis use was greater in part 2 (84% versus 75%), and the upper age limit imposed in part 2 resulted in a younger sample (mean age in part 1 was 69 versus 64 in part 2). In the combined sample, the proportions of good outcome in the 2 treatment groups were identical (41%). Similar results were observed in all secondary efficacy outcomes. Pulmonary edema was a consistent safety concern, with a 6-fold increase in the albumin arm (13%) compared with saline (2%; relative risk =7.76, 95% confidence interval 3.87-15.57).
Conclusions: Treatment with intravenous albumin 25% at 2 g/kg was not associated with improved outcome at 90 days and was associated with increased rates of intracerebral hemorrhage and pulmonary edema.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT00235495.
Keywords: acute stroke; albumin; randomized trial; risk; serum albumin.
© 2016 American Heart Association, Inc.
Figures
Figure 1
Cumulative Efficacy: Parts 1 and 2 Combined
Figure 2
Distribution of 90-day MRS Scores by Treatment
Figure 3
Impact of Treatment on Safety Events by Trial Part
Similar articles
- The Albumin in Acute Stroke Part 1 Trial: an exploratory efficacy analysis.
Hill MD, Martin RH, Palesch YY, Tamariz D, Waldman BD, Ryckborst KJ, Moy CS, Barsan WG, Ginsberg MD; ALIAS Investigators; Neurological Emergencies Treatment Trials Network. Hill MD, et al. Stroke. 2011 Jun;42(6):1621-5. doi: 10.1161/STROKEAHA.110.610980. Epub 2011 May 5. Stroke. 2011. PMID: 21546491 Free PMC article. Clinical Trial. - The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke--II: neurologic outcome and efficacy analysis.
Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD. Palesch YY, et al. Stroke. 2006 Aug;37(8):2107-14. doi: 10.1161/01.STR.0000231389.34701.b5. Epub 2006 Jun 29. Stroke. 2006. PMID: 16809570 - Recombinant tissue-type plasminogen activator plus eptifibatide versus recombinant tissue-type plasminogen activator alone in acute ischemic stroke: propensity score-matched post hoc analysis.
Adeoye O, Sucharew H, Khoury J, Tomsick T, Khatri P, Palesch Y, Schmit PA, Pancioli AM, Broderick JP; CLEAR-ER, IMS III, and ALIAS Part 2 Investigators. Adeoye O, et al. Stroke. 2015 Feb;46(2):461-4. doi: 10.1161/STROKEAHA.114.006743. Epub 2014 Dec 18. Stroke. 2015. PMID: 25523054 Free PMC article. Clinical Trial. - Albumin therapy for acute ischemic stroke: a meta-analysis.
Huang Y, Xiao Z. Huang Y, et al. Neurol Sci. 2021 Jul;42(7):2713-2719. doi: 10.1007/s10072-021-05244-9. Epub 2021 May 4. Neurol Sci. 2021. PMID: 33945037 Review. - Use of albumin in the intensive care unit.
Dubois MJ, Vincent JL. Dubois MJ, et al. Curr Opin Crit Care. 2002 Aug;8(4):299-301. doi: 10.1097/00075198-200208000-00005. Curr Opin Crit Care. 2002. PMID: 12386489 Review.
Cited by
- Elevated Albumin to Globulin Ratio on Day 7 is Associated with Improved Function Outcomes in Acute Ischemic Stroke Patients with Intravenous Thrombolysis.
Yang D, Shen J, Huang H, Wang J, Sun F, Zeng T, Qiu H, Xie H, Chen Y, Li S, Chen Y, Chen G, Weng Y. Yang D, et al. J Inflamm Res. 2022 Apr 26;15:2695-2705. doi: 10.2147/JIR.S347026. eCollection 2022. J Inflamm Res. 2022. PMID: 35505797 Free PMC article. - Efficacy of Novel Carbon Nanoparticle Antioxidant Therapy in a Severe Model of Reversible Middle Cerebral Artery Stroke in Acutely Hyperglycemic Rats.
Fabian RH, Derry PJ, Rea HC, Dalmeida WV, Nilewski LG, Sikkema WKA, Mandava P, Tsai AL, Mendoza K, Berka V, Tour JM, Kent TA. Fabian RH, et al. Front Neurol. 2018 Apr 9;9:199. doi: 10.3389/fneur.2018.00199. eCollection 2018. Front Neurol. 2018. PMID: 29686642 Free PMC article. - Predictive Value of Globulin to Prealbumin Ratio for 3-Month Functional Outcomes in Acute Ischemic Stroke Patients.
Li C, Yang C, Zhu J, Huang H, Zheng J, Hu X, Pan W, Sun F, Zeng T, Qiu H, Jiang Z, Chen Y, Chen Y, Chen G, Weng Y. Li C, et al. Dis Markers. 2022 Mar 17;2022:1120192. doi: 10.1155/2022/1120192. eCollection 2022. Dis Markers. 2022. PMID: 35340417 Free PMC article. - Major complications associated with unfavorable outcome in right-sided large hemisphere infarctions: A single-center study.
Li J, Zhang P, Chen H, Liu Y, Luo X, Zhou J, Wang C. Li J, et al. Brain Behav. 2023 Jul;13(7):e3095. doi: 10.1002/brb3.3095. Epub 2023 Jun 7. Brain Behav. 2023. PMID: 37287379 Free PMC article. - Prognostic Value of Serum Albumin at Admission for Neurologic Outcome with Targeted Temperature Management after Cardiac Arrest.
Kim SH, Youn CS, Kim HJ, Choi SP. Kim SH, et al. Emerg Med Int. 2019 Sep 2;2019:6132542. doi: 10.1155/2019/6132542. eCollection 2019. Emerg Med Int. 2019. PMID: 31565439 Free PMC article.
References
- Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: Marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32:553–560. - PubMed
- Ginsberg M, Belayev L, Bazan N, Marcheselli V, Hill M, Palesch Y, et al. Albumin-based neurotherapeutics for acute ischemic stroke: From bench to bedside. In: J K, S K, editors. Pharmacology of cerebral ischemia. Medpharm Scientific Publishers; Stuttgart: 2004. pp. 421–433.
- Belayev L, Zhao W, Pattany PM, Weaver RG, Huh PW, Lin B, et al. Diffusion-weighted magnetic resonance imaging confirms marked neuroprotective efficacy of albumin therapy in focal cerebral ischemia. Stroke. 1998;29:2587–2599. - PubMed
- Ginsberg MD, Palesch YY, Hill MD. The ALIAS (ALbumin In Acute Stroke) Phase III randomized multicentre clinical trial: design and progress report. Biochem Soc Trans. 2006;34:1323–6. - PubMed
- Hill MD, Moy CS, Palesch YY, Martin R, Dillon CR, Waldman BD, et al. for the ALIAS Investigators The albumin in acute stroke trial (ALIAS); design and methodology. Int J Stroke. 2007;2:214–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical