A small molecule inhibitor of mutant IDH2 rescues cardiomyopathy in a D-2-hydroxyglutaric aciduria type II mouse model - PubMed (original) (raw)
. 2016 Nov;39(6):807-820.
doi: 10.1007/s10545-016-9960-y. Epub 2016 Jul 28.
Jeremy Travins 1, Zhizhong Lin 2, Yaguang Si 1, Yue Chen 1, Josh Powe 1, Stuart Murray 1, Dongwei Zhu 1, Erin Artin 1, Stefan Gross 1, Stephanie Santiago 1, Mya Steadman 1, Andrew Kernytsky 1, Kimberly Straley 1, Chenming Lu 2, Ana Pop 3, Eduard A Struys 3, Erwin E W Jansen 3, Gajja S Salomons 3, Muriel D David 4 5, Cyril Quivoron 4 5, Virginie Penard-Lacronique 4 5, Karen S Regan 6, Wei Liu 1, Lenny Dang 1, Hua Yang 1, Lee Silverman 1, Samuel Agresta 1, Marion Dorsch 1, Scott Biller 1, Katharine Yen 1, Yong Cang 2, Shin-San Michael Su 1, Shengfang Jin 7
Affiliations
- PMID: 27469509
- PMCID: PMC5065612
- DOI: 10.1007/s10545-016-9960-y
A small molecule inhibitor of mutant IDH2 rescues cardiomyopathy in a D-2-hydroxyglutaric aciduria type II mouse model
Fang Wang et al. J Inherit Metab Dis. 2016 Nov.
Abstract
D-2-hydroxyglutaric aciduria (D2HGA) type II is a rare neurometabolic disorder caused by germline gain-of-function mutations in isocitrate dehydrogenase 2 (IDH2), resulting in accumulation of D-2-hydroxyglutarate (D2HG). Patients exhibit a wide spectrum of symptoms including cardiomyopathy, epilepsy, developmental delay and limited life span. Currently, there are no effective therapeutic interventions. We generated a D2HGA type II mouse model by introducing the Idh2R140Q mutation at the native chromosomal locus. Idh2R140Q mice displayed significantly elevated 2HG levels and recapitulated multiple defects seen in patients. AGI-026, a potent, selective inhibitor of the human IDH2R140Q-mutant enzyme, suppressed 2HG production, rescued cardiomyopathy, and provided a survival benefit in Idh2R140Q mice; treatment withdrawal resulted in deterioration of cardiac function. We observed differential expression of multiple genes and metabolites that are associated with cardiomyopathy, which were largely reversed by AGI-026. These findings demonstrate the potential therapeutic benefit of an IDH2R140Q inhibitor in patients with D2HGA type II.
Conflict of interest statement
None. Animal rights All institutional and national guidelines for the care and use of laboratory animals were followed. This article does not contain studies with human subjects performed by any of the authors. Funding This work was funded by Agios. Writing assistance was provided by Helen Varley, PhD, CMPP, Excel Scientific Solutions, Horsham, UK and funded by Agios.
Figures
Fig. 1
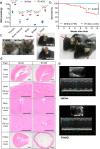
Characteristics of Idh2R140Q KI mice. (a) 2HG levels in plasma (nmol/mL), bone marrow, brain, spleen (all n = 4), and heart (n = 7 for Idh2wt and n = 5 for Idh2R140Q) in Idh2R140Q and Idh2wt mice. Black horizontal bars indicate mean. Statistical significance (p) calculated with t tests using the Holm-Sidak method (p values shown above brackets). (b) Kaplan-Meier survival curves in Idh2R140Q and Idh2wt mice from week 3. Statistical significance (p) tested using the log-rank (Mantel-Cox) test. (c) Abnormal phenotypes (red arrow) in Idh2R140Q mice: runting, facial dysmorphism, and abnormal shape of the head. (d) Histology of the heart, brain, and kidney in Idh2R140Q mice at approximately 12 weeks of age (hematoxylin and eosin stained). Longitudinal sections of the whole heart demonstrate ventricle wall thickening, and cardiomyocytes show hypertrophy. Transverse sections of the mid-brain showed vacuoles were consistently present in the lateral septal nuclei, various strata of the CA1 and CA3 areas of the hippocampus, the dentate gyrus, and the anterior cingulate and retrosplenial cortices, but were also observed laterally and ventrally in the cerebrum to involve the motor (shown), somatosensory and piriform cortices, particularly in the deeper neuronal layers (3 − 6), and were also sometimes present in the amygdala and medulla. Transverse sections of the whole kidney illustrate hydronephrosis, showing thinning of the cortex and almost total loss of the medulla. Histology is representative of six mice per group. (e) Echocardiographs demonstrating cardiac functional defects in 12-week old Idh2R140Q mice
Fig. 2
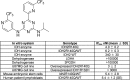
AGI-026, a brain-penetrant, potent and selective IDH2R140Q mutant inhibitor. Structure and biochemical properties of AGI-026. IC50 for 2HG production was measured in vitro for IDH2R140Q homodimer and IDH2wt/R140Q heterodimer recombinant purified enzymes. IC50 for αKG production was measured in vitro for IDH2wt homodimer recombinant purified enzyme. IC50 for 2HG production was measured in: the engineered glioblastoma U87MG cell line overexpressing IDH2R140Q or IDH1R132H; mouse Idh2R140Q embryonic stem cells; lymphoblast cells obtained from three unrelated patients with D2HGA type II. Mouse Idh2R140Q and human IDH2R140Q show 95.13 % identity
Fig. 3
Efficacy of AGI-026 in reversing the phenotype observed in Idh2R140Q mice. Treatment was administered twice daily for 13 weeks. (a) Inhibition of 2HG in plasma and heart following 6 weeks’ treatment with AGI-026 10 mg/kg compared with vehicle (n = 8 for plasma, n = 7 for heart Idh2wt-veh, and n = 5 for other groups). (b) Kaplan-Meier survival curves in Idh2R140Q mice treated with 10 mg/kg AGI-026 or vehicle and Idh2wt mice receiving vehicle. Statistical significance (p) tested using the log-rank (Mantel-Cox) test. (c) Histopathological analysis of heart tissue in Idh2wt mice and Idh2R140Q mice with and without AGI-026 treatment, showing rescue of cardiac hypertrophy (cytoplasmic and nuclear enlargement) with treatment (scale bar denotes 100 μm). (d) Inhibition of 2HG in plasma following 6 weeks’ treatment with AGI-026 compared with vehicle in the second efficacy study (n = 20 for Idh2wt-veh, n = 21 for Idh2R140Q-veh, n = 14 for Idh2R140Q-AGI 2 mg/kg, and n = 10 for Idh2R140Q-AGI 10 mg/kg); % inhibition calculated as 100 % in Idh2wt-veh and 0 % in Idh2R140Q-veh. (e) Kaplan-Meier survival curves in Idh2R140Q mice treated with 2 mg/kg AGI-026, 10 mg/kg AGI-026 or vehicle and Idh2wt mice receiving vehicle. Statistical significance (p) tested using the log-rank (Mantel-Cox) test. (f) Left ventricular mass/body weight ratio and (g) ejection fraction assessed by echocardiogram at week 13 (n = 20 for Idh2wt-veh, n = 16 for Idh2R140Q-veh, n = 13 for Idh2R140Q-AGI 2 mg/kg, and n = 9 for Idh2R140Q-AGI 10 mg/kg). Error bars represent mean ± standard deviation; statistical significance (p) was tested with one-way ANOVA with Sidak’s multiple comparison test
Fig. 4
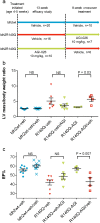
Cross-over study to determine effects of withdrawal of AGI-026 treatment. (a) Cross-over study design. (b) Left ventricular mass/body weight and (c) ejection fraction before and after cross-over study treatment (n = 9 in Idh2wt, n = 7 in Idh2R140Q-veh/AGI, and n = 4 in Idh2R140Q-AGI/veh). Error bars represent mean ± standard deviation. Statistical significance (p) tested with one-way ANOVA with Sidak’s multiple comparison test
Fig. 5
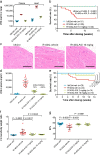
5hmC and 5mC levels in heart tissue of mice. Percentage of 5hmc (a) or 5mc (b) in hearts of Idh2wt mice receiving vehicle (n = 10) and Idh2R140Q mice treated for 13 weeks with vehicle (n = 5) or AGI-026 10 mg/kg (n = 10). Error bars represent mean ± standard deviation. Statistical significance (p) was tested by one-way ANOVA with Sidak’s multiple comparison test
Fig. 6
Differences in metabolite levels and gene expression in Idh2R140Q and Idh2wt heart tissue and effect of AGI-026. For heat maps (a) and (c), red indicates an increase, blue a decrease, darker colors indicate greater change, and pale colors indicate less change. Left column shows difference for Idh2R140Q versus Idh2wt, central column shows difference for AGI-026 versus vehicle treatment in Idh2R140Q mice, and right column shows difference for AGI-026-treated Idh2R140Q versus Idh2wt mice (pale colors illustrate correction of alterations by AGI-026 and shift to Idh2wt status). (a) Expression changes in 52 candidate genes with ≥ 2-fold difference between Idh2R140Q (n = 6) and Idh2wt (n = 5); (Idh2R140Q-AGI n = 6). (b) Quantitative RT-PCR validation of expression changes in two candidate genes, shown as relative expression normalized to one Idh2wt sample. Each data point represents the mean of a technical triplicate from each of the five animals per group. P tested using Kruskal-Wallis tests with Dunn’s multiple comparison test. For all figures horizontal black bars indicate group means. (c) The metabolites (excluding 2HG) that were significantly changed (p < 0.05) in heart tissue of Idh2R140Q versus Idh2wt mice, and effects of AGI-026. (d) Levels of cAMP and metabolites in the glycogenolysis and glycolysis pathways that are corrected by AGI-026 treatment. Statistical significance (p) was tested with one-way ANOVA with Sidak’s multiple comparison test (n = 10 Idh2wt-veh and Idh2R140Q-AGI, n = 5 Idh2R140Q-veh). (e) Correlation of fold-changes for Idh2R140Q-AGI versus Idh2R140Q-veh against Idh2R140Q-veh versus Idh2wt-veh for the metabolites in heart tissue significantly altered by Idh2R140Q expression (excluding 2HG). Red dots indicate metabolites significantly changed on both axes (n = 32). R2 denotes coefficient of determination
Similar articles
- Enasidenib treatment in two individuals with D-2-hydroxyglutaric aciduria carrying a germline IDH2 mutation.
Geoerger B, Schiff M, Penard-Lacronique V, Darin N, Saad SM, Duchon C, Lamazière A, Desmons A, Pontoizeau C, Berlanga P, Ducassou S, Yen K, Su M, Schenkein D, Ottolenghi C, De Botton S. Geoerger B, et al. Nat Med. 2023 Jun;29(6):1358-1363. doi: 10.1038/s41591-023-02382-9. Epub 2023 May 29. Nat Med. 2023. PMID: 37248298 - D-2-hydroxyglutarate produced by mutant IDH2 causes cardiomyopathy and neurodegeneration in mice.
Akbay EA, Moslehi J, Christensen CL, Saha S, Tchaicha JH, Ramkissoon SH, Stewart KM, Carretero J, Kikuchi E, Zhang H, Cohoon TJ, Murray S, Liu W, Uno K, Fisch S, Jones K, Gurumurthy S, Gliser C, Choe S, Keenan M, Son J, Stanley I, Losman JA, Padera R, Bronson RT, Asara JM, Abdel-Wahab O, Amrein PC, Fathi AT, Danial NN, Kimmelman AC, Kung AL, Ligon KL, Yen KE, Kaelin WG Jr, Bardeesy N, Wong KK. Akbay EA, et al. Genes Dev. 2014 Mar 1;28(5):479-90. doi: 10.1101/gad.231233.113. Genes Dev. 2014. PMID: 24589777 Free PMC article. - IDH2 mutations in patients with D-2-hydroxyglutaric aciduria.
Kranendijk M, Struys EA, van Schaftingen E, Gibson KM, Kanhai WA, van der Knaap MS, Amiel J, Buist NR, Das AM, de Klerk JB, Feigenbaum AS, Grange DK, Hofstede FC, Holme E, Kirk EP, Korman SH, Morava E, Morris A, Smeitink J, Sukhai RN, Vallance H, Jakobs C, Salomons GS. Kranendijk M, et al. Science. 2010 Oct 15;330(6002):336. doi: 10.1126/science.1192632. Epub 2010 Sep 16. Science. 2010. PMID: 20847235 - An overview of L-2-hydroxyglutarate dehydrogenase gene (L2HGDH) variants: a genotype-phenotype study.
Steenweg ME, Jakobs C, Errami A, van Dooren SJ, Adeva Bartolomé MT, Aerssens P, Augoustides-Savvapoulou P, Baric I, Baumann M, Bonafé L, Chabrol B, Clarke JT, Clayton P, Coker M, Cooper S, Falik-Zaccai T, Gorman M, Hahn A, Hasanoglu A, King MD, de Klerk HB, Korman SH, Lee C, Meldgaard Lund A, Mejaski-Bosnjak V, Pascual-Castroviejo I, Raadhyaksha A, Rootwelt T, Roubertie A, Ruiz-Falco ML, Scalais E, Schimmel U, Seijo-Martinez M, Suri M, Sykut-Cegielska J, Trefz FK, Uziel G, Valayannopoulos V, Vianey-Saban C, Vlaho S, Vodopiutz J, Wajner M, Walter J, Walter-Derbort C, Yapici Z, Zafeiriou DI, Spreeuwenberg MD, Celli J, den Dunnen JT, van der Knaap MS, Salomons GS. Steenweg ME, et al. Hum Mutat. 2010 Apr;31(4):380-90. doi: 10.1002/humu.21197. Hum Mutat. 2010. PMID: 20052767 Review. - Progress in understanding 2-hydroxyglutaric acidurias.
Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS, Jakobs C. Kranendijk M, et al. J Inherit Metab Dis. 2012 Jul;35(4):571-87. doi: 10.1007/s10545-012-9462-5. Epub 2012 Mar 6. J Inherit Metab Dis. 2012. PMID: 22391998 Free PMC article. Review.
Cited by
- Advances in Chiral Metabolomic Profiling and Biomarker Discovery.
Pandey R, Tiziani S. Pandey R, et al. Methods Mol Biol. 2025;2855:85-101. doi: 10.1007/978-1-0716-4116-3_5. Methods Mol Biol. 2025. PMID: 39354302 Review. - Enasidenib treatment in two individuals with D-2-hydroxyglutaric aciduria carrying a germline IDH2 mutation.
Geoerger B, Schiff M, Penard-Lacronique V, Darin N, Saad SM, Duchon C, Lamazière A, Desmons A, Pontoizeau C, Berlanga P, Ducassou S, Yen K, Su M, Schenkein D, Ottolenghi C, De Botton S. Geoerger B, et al. Nat Med. 2023 Jun;29(6):1358-1363. doi: 10.1038/s41591-023-02382-9. Epub 2023 May 29. Nat Med. 2023. PMID: 37248298 - Metabolism, Activity, and Targeting of D- and L-2-Hydroxyglutarates.
Ye D, Guan KL, Xiong Y. Ye D, et al. Trends Cancer. 2018 Feb;4(2):151-165. doi: 10.1016/j.trecan.2017.12.005. Epub 2018 Jan 5. Trends Cancer. 2018. PMID: 29458964 Free PMC article. Review. - Isocitrate dehydrogenase mutations in myeloid malignancies.
Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R. Medeiros BC, et al. Leukemia. 2017 Feb;31(2):272-281. doi: 10.1038/leu.2016.275. Epub 2016 Oct 10. Leukemia. 2017. PMID: 27721426 Free PMC article. Review. - Symptomatic Heart Failure in Acute Leukemia Patients Treated With Anthracyclines.
Kang Y, Assuncao BL, Denduluri S, McCurdy S, Luger S, Lefebvre B, Carver J, Scherrer-Crosbie M. Kang Y, et al. JACC CardioOncol. 2019 Dec;1(2):208-217. doi: 10.1016/j.jaccao.2019.10.008. Epub 2019 Dec 17. JACC CardioOncol. 2019. PMID: 32905430 Free PMC article.
References
- Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous