Decreased expression of SLC 39A14 is associated with tumor aggressiveness and biochemical recurrence of human prostate cancer - PubMed (original) (raw)
Decreased expression of SLC 39A14 is associated with tumor aggressiveness and biochemical recurrence of human prostate cancer
Xiao-Ming Xu et al. Onco Targets Ther. 2016.
Abstract
Objective: Solute carrier family 39, member 14 (SLC39A14), has been identified as a potential biomarker for various cancers. However, its roles in prostate cancer (PCa) are still unclear. The aim of this study was to investigate the clinical significance of SLC39A14 in patients with PCa and its functions in malignant phenotypes of PCa cells.
Patients and methods: Subcellular localization and expression pattern of SLC39A14 protein were examined by immunohistochemistry. Then, the associations of SLC39A14 expression with various clinicopathological features and clinical outcome of patients with PCa were statistically evaluated. Subsequently, the effects of SLC39A14 overexpression and knockdown on PCa cell proliferation and motility were, respectively, examined by Cell Counting Kit-8, transwell, and wound-healing assays.
Results: The immunoreactive scores of SLC39A14 protein in human PCa tissues were significantly lower than those in normal prostate tissues. Based on the Taylor dataset, SLC39A14 downregulation occurred more frequently in patients with PCa with a higher Gleason score (P<0.001), advanced clinical stage (P=0.008), presence of metastasis (P=0.009), and prostate-specific antigen failure (P=0.006). More interestingly, the survival analysis identified SLC39A14 as an independent factor for predicting the biochemical recurrence-free survival of patients with PCa (P=0.017). Functionally, the enforced expression of SLC39A14 could suppress cell proliferation, invasion, and migration of PCa cell lines in vitro, which could be reversed by the knockdown of SLC39A14.
Conclusion: Decreased expression of SLC39A14 may lead to malignant phenotypes of PCa cells and aggressive tumor progression in patients with PCa. Importantly, SLC39A14 may function as a tumor suppressor and a biomarker for screening patients with biochemical recurrence following radical prostatectomy.
Keywords: biochemical recurrence-free survival; prostate cancer; solute carrier family 39 member 14; tumor suppressor.
Figures
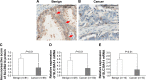
Figure 1
Decreased expression of SLC39A14 protein and mRNA in human PCa tissues. Notes: (A) SLC39A14 protein was mainly localized in the membrane and cytoplasm of prostate cells in adjacent noncancerous prostate tissues. Red arrows show strong positive immunostrainings. Magnification, ×400. (B) SLC39A14 protein was weakly expressed in cancer cells in PCa tissues. Magnification, ×400. (C) Statistical analysis revealed that SLC39A14 protein expression levels in human PCa tissues were significantly lower than those in adjacent noncancerous prostate tissues (P<0.01). (D) SLC39A14 mRNA expression levels in human PCa tissues were also lower than those in adjacent noncancerous prostate tissues based on the Taylor dataset (P<0.01). (E) SLC39A14 mRNA expression levels in human PCa tissues were also lower than those in adjacent noncancerous prostate tissues based on our clinical samples (P<0.01). Abbreviations: PCa, prostate cancer; SLC39A14, solute carrier family 39, member 14.
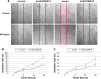
Figure 2
Kaplan–Meier curves of patients with PCa based on SLC39A14 mRNA expression. Notes: (A) There was a significant difference in BCR-free survival between patients with high and low SLC39A14 mRNA expression (_P_=0.017). (B) There was no significant difference in overall survival between patients with high and low SLC39A14 mRNA expression (_P_=0.148). Abbreviations: BCR, biochemical recurrence; PCa, prostate cancer; SLC39A14, solute carrier family 39, member 14.
Figure 3
Downregulation of SLC39A14 promotes cell proliferation of LNCaP cells in vitro. Notes: (A) Western blot analysis showed that SLC39A14 protein expression was significantly upregulated by the transfection of SLC39A14 plasmid (en-SLC39A14 or en-con), but was significantly downregulated by the transfection of SLC39A14 siRNA (si-SLC39A14 or si-con). (B) CCK-8 assay indicated that the cell viability of LNCaP cells with overexpression of SLC39A14 was significantly lower than those of control vector-transfected cells. (C) CCK-8 assay indicated that the cell viability of LNCaP cells with knockdown of SLC39A14 dramatically promoted the cell viability. Abbreviations: CCK-8, Cell Counting Kit-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; SLC39A14, solute carrier family 39, member 14; OD, optical density.
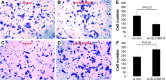
Figure 4
Downregulation of SLC39A14 promotes invasion of LNCaP cells in vitro. Notes: Transwell assays clearly revealed that downregulation of SLC39A14 significantly enhanced the invasion activity of LNCaP cells compared to that of control cells at 24 hours after the transfection, while overexpression of SLC39A14 dramatically reduced the cell invasion. Cell invasion of LNCaP cells transfected with en-con (A), en-SLC39A14 (B), si-con (C) and si-SLC39A14 (D). (E) The number of invasive cells after the transfection of en-con and en-SLC39A14. (F) The number of invasive cells after the transfection of si-con and si-SLC39A14. Abbreviation: SLC39A14, solute carrier family 39, member 14.
Figure 5
Downregulation of SLC39A14 promotes migration of LNCaP cells in vitro. Notes: (A) Cell migration of LNCaP cells transfected with en-con, en-SLC39A14, si-con and si-SLC39A14. (B) Inhibition rate of LNCaP cells after the transfection of en-con and en-SLC39A14. (C) Inhibition rate of LNCaP cells after the transfection of si-con and si-SLC39A14. Wound-healing assays demonstrated that downregulation of SLC39A14 significantly enhanced the migration activity of LNCaP cells compared to that of control cells at 24 hours after the transfection, while overexpression of SLC39A14 dramatically reduced the cell migration. Abbreviation: SLC39A14, solute carrier family 39, member 14.
Similar articles
- High expression of Rab25 contributes to malignant phenotypes and biochemical recurrence in patients with prostate cancer after radical prostatectomy.
Hu C, Chen B, Zhou Y, Shan Y. Hu C, et al. Cancer Cell Int. 2017 Apr 11;17:45. doi: 10.1186/s12935-017-0411-0. eCollection 2017. Cancer Cell Int. 2017. PMID: 28400705 Free PMC article. - HMGCS2 functions as a tumor suppressor and has a prognostic impact in prostate cancer.
Wan S, Xi M, Zhao HB, Hua W, Liu YL, Zhou YL, Zhuo YJ, Liu ZZ, Cai ZD, Wan YP, Zhong WD. Wan S, et al. Pathol Res Pract. 2019 Aug;215(8):152464. doi: 10.1016/j.prp.2019.152464. Epub 2019 May 22. Pathol Res Pract. 2019. PMID: 31176575 - Overexpression of SLC6A1 associates with drug resistance and poor prognosis in prostate cancer.
Chen C, Cai Z, Zhuo Y, Xi M, Lin Z, Jiang F, Liu Z, Wan Y, Zheng Y, Li J, Zhou X, Zhu J, Zhong W. Chen C, et al. BMC Cancer. 2020 Apr 6;20(1):289. doi: 10.1186/s12885-020-06776-7. BMC Cancer. 2020. PMID: 32252682 Free PMC article. - ARRDC3 Inhibits the Progression of Human Prostate Cancer Through ARRDC3-ITGβ4 Pathway.
Zheng Y, Lin ZY, Xie JJ, Jiang FN, Chen CJ, Li JX, Zhou X, Zhong WD. Zheng Y, et al. Curr Mol Med. 2017;17(3):221-229. doi: 10.2174/1566524017666170807144711. Curr Mol Med. 2017. PMID: 28782483 - Decreased expression of TCF12 contributes to progression and predicts biochemical recurrence in patients with prostate cancer.
Chen QB, Liang YK, Zhang YQ, Jiang MY, Han ZD, Liang YX, Wan YP, Yin J, He HC, Zhong WD. Chen QB, et al. Tumour Biol. 2017 Jun;39(6):1010428317703924. doi: 10.1177/1010428317703924. Tumour Biol. 2017. PMID: 28651494
Cited by
- A guide to plasma membrane solute carrier proteins.
Pizzagalli MD, Bensimon A, Superti-Furga G. Pizzagalli MD, et al. FEBS J. 2021 May;288(9):2784-2835. doi: 10.1111/febs.15531. Epub 2020 Sep 18. FEBS J. 2021. PMID: 32810346 Free PMC article. Review. - Evaluation of the prognostic values of solute carrier (SLC) family 39 genes for patients with lung adenocarcinoma.
Zhou H, Zhu Y, Qi H, Liang L, Wu H, Yuan J, Hu Q. Zhou H, et al. Aging (Albany NY). 2021 Feb 1;13(4):5312-5331. doi: 10.18632/aging.202452. Epub 2021 Feb 1. Aging (Albany NY). 2021. PMID: 33535184 Free PMC article. - Human-like hyperplastic prostate with low ZIP1 induced solely by Zn deficiency in rats.
Fong LY, Jing R, Smalley KJ, Wang ZX, Taccioli C, Fan S, Chen H, Alder H, Huebner K, Farber JL, Fiehn O, Croce CM. Fong LY, et al. Proc Natl Acad Sci U S A. 2018 Nov 20;115(47):E11091-E11100. doi: 10.1073/pnas.1813956115. Epub 2018 Nov 5. Proc Natl Acad Sci U S A. 2018. PMID: 30397150 Free PMC article. - Roles and therapeutic potential of the SLC family in prostate cancer-literature review.
Fu Y, Chen J, Zhu X, Ding M, Wang H, Fu S. Fu Y, et al. BMC Urol. 2025 Feb 18;25(1):32. doi: 10.1186/s12894-025-01714-w. BMC Urol. 2025. PMID: 39966814 Free PMC article. Review. - Ironomycin induces mantle cell lymphoma cell death by targeting iron metabolism addiction.
Ovejero S, Alibert L, Devin J, Cañeque T, Jacquier V, Romero A, Amar S, Abouladze M, de Paco EG, Gadacha OK, Requirand G, Robert N, Zellagui ML, de Boussac H, Cartron G, Chiche J, Ricci JE, Herbaux C, Rodriguez R, Moreaux J, Bret C. Ovejero S, et al. Theranostics. 2025 Feb 3;15(7):2834-2851. doi: 10.7150/thno.101821. eCollection 2025. Theranostics. 2025. PMID: 40083931 Free PMC article.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. - PubMed
- Molitierno J, Evans A, Mohler JL, Wallen E, Moore D, Pruthi RS. Characterization of biochemical recurrence after radical prostatectomy. Urol Int. 2006;77(2):130–134. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources