Amyloid-like Self-Assembly of a Cellular Compartment - PubMed (original) (raw)
Amyloid-like Self-Assembly of a Cellular Compartment
Elvan Boke et al. Cell. 2016.
Abstract
Most vertebrate oocytes contain a Balbiani body, a large, non-membrane-bound compartment packed with RNA, mitochondria, and other organelles. Little is known about this compartment, though it specifies germline identity in many non-mammalian vertebrates. We show Xvelo, a disordered protein with an N-terminal prion-like domain, is an abundant constituent of Xenopus Balbiani bodies. Disruption of the prion-like domain of Xvelo, or substitution with a prion-like domain from an unrelated protein, interferes with its incorporation into Balbiani bodies in vivo. Recombinant Xvelo forms amyloid-like networks in vitro. Amyloid-like assemblies of Xvelo recruit both RNA and mitochondria in binding assays. We propose that Xenopus Balbiani bodies form by amyloid-like assembly of Xvelo, accompanied by co-recruitment of mitochondria and RNA. Prion-like domains are found in germ plasm organizing proteins in other species, suggesting that Balbiani body formation by amyloid-like assembly could be a conserved mechanism that helps oocytes function as long-lived germ cells.
Keywords: velo1.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
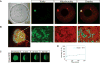
Figure 1. A Balbiani body is a non-membrane bound compartment packed with membranous organelles
(A) Phase contrast image of a stage I Xenopus oocyte. Bb, Balbiani body; N, nucleus, or germinal vesicle. (B) Balbiani body immobilized in perfusion chambers. 2M NaCl (first panel) or 95 °C 50mM Hepes, 100mM KCl, pH 7.6 buffer (second panel) was perfused into the chambers. (C) Thin-section electron microscope (EM) images of isolated Balbiani bodies from stage I Xenopus oocytes. Mitochondria (dark spots), RNP particles (green arrow head) and Golgi stacks (yellow arrow head) are clearly visible. (D) Stage I oocytes were incubated in 10 μM Thioflavin T in 1× MMR for 10 minutes and washed twice with 1X MMR. See also Figure S1, Table S1 and Movie S1
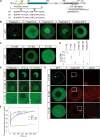
Figure 2. Xvelo forms a stable matrix
(A) mRNA encoding for Xvelo-GFP was microinjected into stage I oocytes. Mitotracker Deep Red was used to label mitochondria. Oocytes were imaged live with a scanning confocal microscope with a 40× Water immersion objective. (B) Magnification of the Balbiani body in (A). (C) Internal rearrangement of fluorescent Xvelo-GFP particles after half bleach over time. (D) The fluorescent recovery of the half-bleached Xvelo-GFP in the Balbiani body in (C) and two other biological replicates are shown by quantification of fluorescence in bleached region over time. Fluorescent intensity changes in the bleached region per pixel over time were plotted after it was normalized for photobleaching by using an unbleached neighbouring area and background subtraction. See also Figure S2
Figure 3. Xvelo self-assembly is dependent on its prion-like domain
(A) Diagram of the known structural elements of Xvelo. Prion-like domain, mutants (4D and 7D) and the fragments of Xvelo (F1 to F4) are marked in the figure. (B) mRNAs encoding for Xvelo fragments shown in (A) and Xvelo without fragment 1 (Xvelo-woF1) are in vitro synthesized and micro-injected into stage I oocytes. Oocytes were imaged after overnight incubation in Oocyte Culture Medium (OCM). (C) mRNAs encoding for wild type and PLD mutants of Fragment1-GFP were microinjected into oocytes. Oocytes were incubated overnight and imaged. (D) Ratio of GFP concentration in the oocyte cytoplasm (Ccy) to the Balbiani body (CBb) in oocytes injected with mRNAs encoding for indicated proteins. Relative concentrations were calculated by using oocyte or the Balbiani body volume from z-stacks and the fluorescent intensity of GFP. Mean values and standard errors of 10 oocytes are plotted. (E) Internal rearrangement of fluorescent wild-type or mutant F1-GFP particles after photobleaching over time. (F) The fluorescent recovery of photobleached wild-type or mutant F1-GFP in Balbiani bodies in (E) and two other biological replicates for each are shown by quantification of fluorescence in bleached region over time normalized by an unbleached neighbouring region. (G) mRNAs encoding for full length Xvelo-mCherry wild-type, and GFP tagged fragments, F1-WT-GFP, F1-4D-GFP and F1-7D-GFP, were in vitro synthesized. F1-WT-GFP or mutants were mixed with equal amounts of full length Xvelo-mCherry mRNA and microinjected into the oocytes. After overnight incubation, the oocytes were imaged by scanning confocal microscopy. See also Figure S3
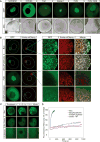
Figure 4. Xvelo forms micron scale networks in vitro
(A) 15 μM of recombinant Xvelo-GFP, F1-WT-GFP, and full length mutants, Xvelo-4D-GFP and Xvelo-7D-GFP, were diluted into a low arginine buffer (30 mM) to promote their self-assembly. The reaction mixtures were incubated at 25 °C for the indicated time intervals, and squashed under a coverslip to be imaged by spinning confocal microscopy. (B) Coomassie stained gels depicting recombinant Xvelo-GFP, Xvelo-4D-GFP, Xvelo-7D-GFP and F1-WT-GFP. (C) Quantification of networks in (A). 20 images were taken and stitched together, a threshold was applied and the network intensities were measured. The integrated intensity of networks per sample at each time point (total network mass) is plotted. Means and standard errors of three biological replicates are shown. See also Figure S4
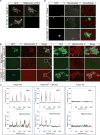
Figure 5. Xvelo shows amyloid-like features in vivo and in vitro
(A) SDS was added to Xvelo and F1-WT-GFP networks to a final 1 % concentration, and the reactions were incubated at room temperature for 15 minutes. The resulting mixtures were squashed under a coverslip and imaged by a spinning-disc confocal microscope. (B) A final concentration of 5 μM of Thioflavin T was added to the wildtype, F1 and mutant network reactions at the indicated time points. Yeast prion Mot3 was used as a positive control, whereas blank was only buffer and ThT. ThT fluorescence was measured (arbitrary units) by a fluorescence plate reader. (C) 1 μg of RFP tagged wild-type, F1 and mutant recombinant proteins were dot-blotted on a nitrocellulose membrane and assayed for reactivity with α-amyloid fibril OC. EB1-RFP was used as a negative control. (D) Negative stain electron microscopy images of the untagged Xvelo-WT and Xvelo-4D self-assembly reactions (Scale bars 100 nm). (E) Stage I oocytes were injected with mRNA coding for Xvelo-mCherry, and incubated overnight. The oocytes were incubated in 10 μM ThT, washed twice and imaged by confocal microscopy. Lower panel: Zoomed in images. Line scans showing the co-localization of Xvelo-mCherry and ThT stain from 5 Balbiani bodies were plotted. Each colour represents the line scan of a different Balbiani body. We speculate that the outer rim Xvelo-mCherry signal belongs to the newly translated Xvelo-mCherry protein that has just started to form a new, immature matrix, and does not yet stain with ThT. (F) SDD-AGE detects SDS-resistant Xvelo aggregates in vivo. Equal amounts of cytoplasmic extracts of stage I oocytes and mature eggs were loaded onto SDS-PAGE. Five times more amount of egg extracts were loaded for SDD-AGE gels to make Xvelo concentrations comparable between the oocyte and egg extract lanes. Xvelo was detected by an anti-Xvelo antibody. See also Figure S5
Figure 6. Xvelo has unique properties for forming a stable matrix
(A) mRNAs encoding for GFP tagged hnRNPA1, CPEB3, Tia1, Dazap1, FUS and FUS156E were in vitro synthesized and microinjected into the oocytes. After overnight incubation, the oocytes were imaged by scanning confocal microscopy. (B) mRNAs encoding for Xvelo-mCherry and GFP tagged Bucky ball (Buc), FUS, and the PLD-swap versions of Xvelo, in which the PLD of Xvelo was replaced either by the PLD of Buckyball (BucPLDXvelo) or the PLD of FUS (FUSPLDXvelo) were injected into the oocytes at equal concentrations and imaged after overnight incubation. (C) Internal rearrangement of fluorescent Buckyball-GFP (Buc), and PLD-swap versions of Xvelo, Buc(PLD)Xvelo-GFP and FUS(PLD)Xvelo-GFP after photobleaching over time. (D) The fluorescent recovery of photobleached constructs in Balbiani bodies in (C), as well as Xvelo-WT, and two other biological replicates for each are shown by quantification of fluorescence in bleached region over time normalized by an unbleached neighbouring region. See also Figure S6
Figure 7. Xvelo-GFP aggregates bind to RNA and cluster mitochondria
(A) Labeled RNAs, Xenopus nanos homolog xcat-2, and an mRNA encoding for mCherry protein were prepared using the MEGAscript SP6 kit with ChromaTide Alexa Fluor 546-14-UTP. RNAs were added to pre-assembled networks and imaged with a spinning-disc confocal microscope. (B) Recombinant Xvelo-WT-GFP or the prion-like domain mutant, Xvelo-4D-GFP were added to Xenopus egg extracts with intact actin. Xvelo-GFP fills the gaps between mitochondria (arrows, compare to Figure 2B). Mitochondria were labeled with MitoTracker Deep Red. Images were taken with a spinning-disc confocal microscope. Lower panel: The extracts were diluted with KCl so that the final KCl concentration was 2M. (C) Line scans of Xvelo-WT-GFP and Xvelo-4D-GFP and mitochondria in Xenopus egg extracts. Five images were stitched together to have an area spanning larger than 2 mm2. Each colour represents a different field. (D) Recombinant FUS-WT-GFP or the aggregation prone mutant, FUS-G156E-GFP were added to Xenopus egg extracts with intact actin. Arrows point to the exclusion zones of mitochondria in presence of FUS structures. See also Figure S7
Similar articles
- Clustering of Aromatic Residues in Prion-like Domains Can Tune the Formation, State, and Organization of Biomolecular Condensates.
Holehouse AS, Ginell GM, Griffith D, Böke E. Holehouse AS, et al. Biochemistry. 2021 Nov 30;60(47):3566-3581. doi: 10.1021/acs.biochem.1c00465. Epub 2021 Nov 16. Biochemistry. 2021. PMID: 34784177 Free PMC article. - The balbiani body and the concept of physiological amyloids.
Boke E, Mitchison TJ. Boke E, et al. Cell Cycle. 2017 Jan 17;16(2):153-154. doi: 10.1080/15384101.2016.1241605. Epub 2016 Oct 13. Cell Cycle. 2017. PMID: 27736303 Free PMC article. No abstract available. - Protein interactions in Xenopus germ plasm RNP particles.
Nijjar S, Woodland HR. Nijjar S, et al. PLoS One. 2013 Nov 12;8(11):e80077. doi: 10.1371/journal.pone.0080077. eCollection 2013. PLoS One. 2013. PMID: 24265795 Free PMC article. - The vertebrate Balbiani body, germ plasm, and oocyte polarity.
Jamieson-Lucy A, Mullins MC. Jamieson-Lucy A, et al. Curr Top Dev Biol. 2019;135:1-34. doi: 10.1016/bs.ctdb.2019.04.003. Epub 2019 May 3. Curr Top Dev Biol. 2019. PMID: 31155356 Review. - Selection of mitochondria in female germline cells: is Balbiani body implicated in this process?
Bilinski SM, Kloc M, Tworzydlo W. Bilinski SM, et al. J Assist Reprod Genet. 2017 Nov;34(11):1405-1412. doi: 10.1007/s10815-017-1006-3. Epub 2017 Jul 28. J Assist Reprod Genet. 2017. PMID: 28755153 Free PMC article. Review.
Cited by
- Keeping up with the condensates: The retention, gain, and loss of nuclear membrane-less organelles.
Lacroix E, Audas TE. Lacroix E, et al. Front Mol Biosci. 2022 Sep 20;9:998363. doi: 10.3389/fmolb.2022.998363. eCollection 2022. Front Mol Biosci. 2022. PMID: 36203874 Free PMC article. Review. - Emerging Implications of Phase Separation in Cancer.
Ren J, Zhang Z, Zong Z, Zhang L, Zhou F. Ren J, et al. Adv Sci (Weinh). 2022 Nov;9(31):e2202855. doi: 10.1002/advs.202202855. Epub 2022 Sep 18. Adv Sci (Weinh). 2022. PMID: 36117111 Free PMC article. Review. - Probing and engineering liquid-phase organelles.
Bracha D, Walls MT, Brangwynne CP. Bracha D, et al. Nat Biotechnol. 2019 Dec;37(12):1435-1445. doi: 10.1038/s41587-019-0341-6. Epub 2019 Dec 2. Nat Biotechnol. 2019. PMID: 31792412 Review. - A Non-amyloid Prion Particle that Activates a Heritable Gene Expression Program.
Chakravarty AK, Smejkal T, Itakura AK, Garcia DM, Jarosz DF. Chakravarty AK, et al. Mol Cell. 2020 Jan 16;77(2):251-265.e9. doi: 10.1016/j.molcel.2019.10.028. Epub 2019 Nov 19. Mol Cell. 2020. PMID: 31757755 Free PMC article. - The (un)structural biology of biomolecular liquid-liquid phase separation using NMR spectroscopy.
Murthy AC, Fawzi NL. Murthy AC, et al. J Biol Chem. 2020 Feb 21;295(8):2375-2384. doi: 10.1074/jbc.REV119.009847. Epub 2020 Jan 7. J Biol Chem. 2020. PMID: 31911439 Free PMC article. Review.
References
- Al-Mukhtar KA, Webb AC. An ultrastructural study of primordial germ cells, oogonia and early oocytes in Xenopus laevis. Journal of embryology and experimental morphology. 1971;26:195–217. - PubMed
- Alberti S, Halfmann R, Lindquist S. Biochemical, cell biological, and genetic assays to analyze amyloid and prion aggregation in yeast. Methods in enzymology. 2010;470:709–734. - PubMed
- Bagriantsev SN, Kushnirov VV, Liebman SW. Analysis of amyloid aggregates using agarose gel electrophoresis. Methods in enzymology. 2006;412:33–48. - PubMed
- Balinsky B, Devis RJ. Origin and differentiation of cytoplasmic structures in the oocytes of Xenopus laevis. Acta Embryol Morphol Exp. 1963;6:55–108.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM039565/GM/NIGMS NIH HHS/United States
- R01 GM103785/GM/NIGMS NIH HHS/United States
- R01 HD091846/HD/NICHD NIH HHS/United States
- R37 GM039565/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases