Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats - PubMed (original) (raw)
Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats
Mingming Zhang et al. BMC Gastroenterol. 2016.
Abstract
Background: Butyrate is an energy source for colonocytes that is formed by bacterial fermentation of dietary fiber in the colon and that exerts broad anti-inflammatory activities. Although the administration of butyrate improves homeostasis in patients and ameliorates IBD (Inflammatory Bowel Disease)-related lesions and symptoms, the anti-inflammatory mechanisms of butyrate still remain unclear. To explore the impact of butyrate on Treg (Regulatory T cell)/Th17 (T helper 17 cell) differentiation and colitis in rats.
Methods: The effect of butyrate on the expression of markers related to both Tregs and Th17 cells were determined in human monocytes as well as a rat model of colitis induced by 2,4,6-trinitrobenzene sulfonic acid. Rats were treated with butyrate in vivo, whereas the rat splenocytes and human monocytes were treated in vitro.
Results: We found that butyrate administration increased peripheral blood Treg cell levels as well as plasma levels of anti-Th17 cytokines (IL-10 and IL-12). Butyrate administration further suppressed IL-17 levels in both plasma and colonic mucosa, and ameliorated colonic colitis lesions in rats. This promotion of Treg activity and inhibition of IL-17 release was also observed in human venous monocytes and rat splenocytes in vitro.
Conclusions: Our results suggest that butyrate plays a key role in regulating the Treg/Th17 balance and ultimately protects the colon mucosa against the development of IBD.
Keywords: Butyrate; Cytokines; Inflammatory bowel disease; Th17; Treg.
Figures
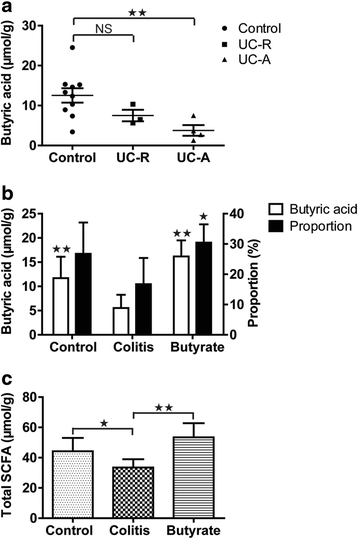
Fig. 1
Intestinal fatty acid levels. Human fecal butyrate concentration (a). Rat fecal butyric acid concentration and percentage of total SCFA content (b). Rat fecal total SCFA content (c). Data are the mean ± SE. n = 5–7. *P < 0.05; **P < 0.01; NS: No Significance. UC-R, remission phase of ulcerative colitis; active phase of ulcerative colitis
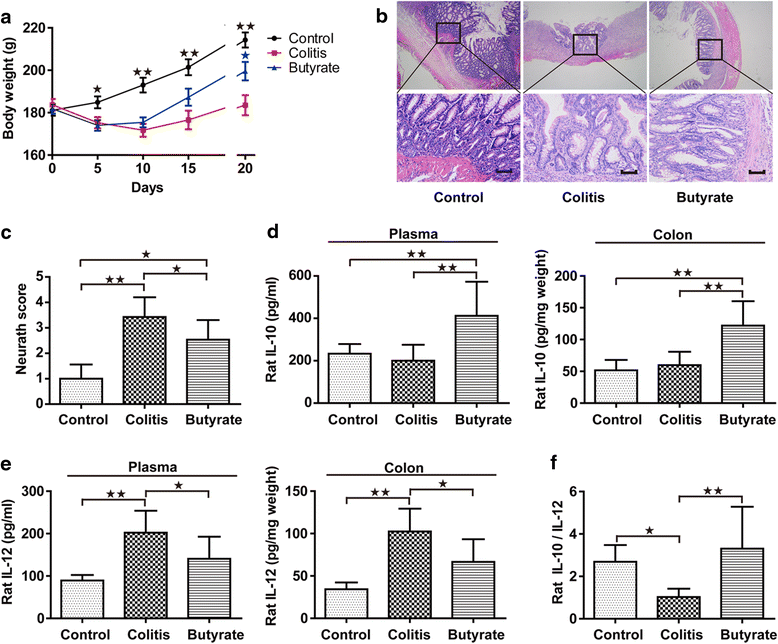
Fig. 2
Body weight, colon histology and cytokines in rats. Body weight (a). Colon histology, upper and lower panel magnifications are × 40 and × 200, respectively. Scale bars, 200 μm (b). Colon Neurath score (c). IL-10 of plasma and colon (d). IL-12 of plasma and colon (e). Plasma IL-10/IL-12 (f). Data are the mean ± SE. n = 5–7. *P < 0.05; **P < 0.01
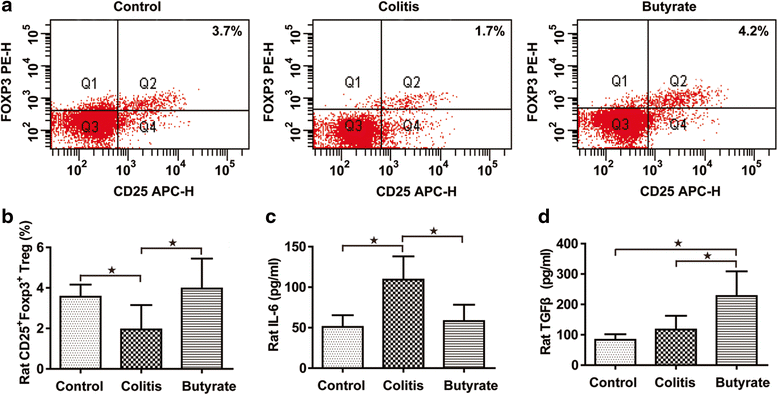
Fig. 3
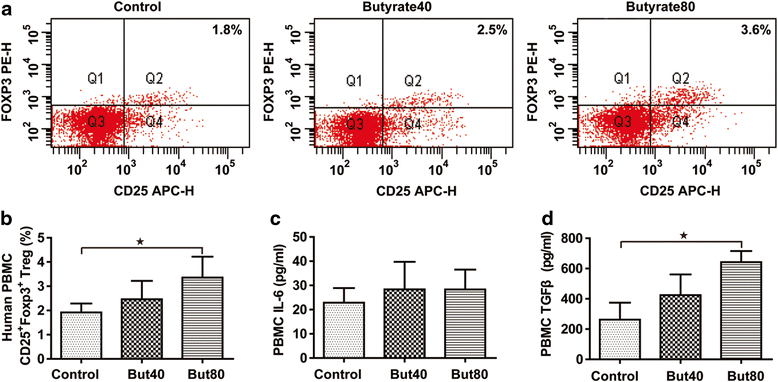
Treg analysis in rats. The percentage of CD4+CD25+Foxp3+ Tregs (a). Quantified CD4+CD25+Foxp3+ Tregs (b). Plasma IL-6 (c). Plasma TGF-β (d). Data are the mean ± SE. n = 5–7. *P < 0.05; **P < 0.01
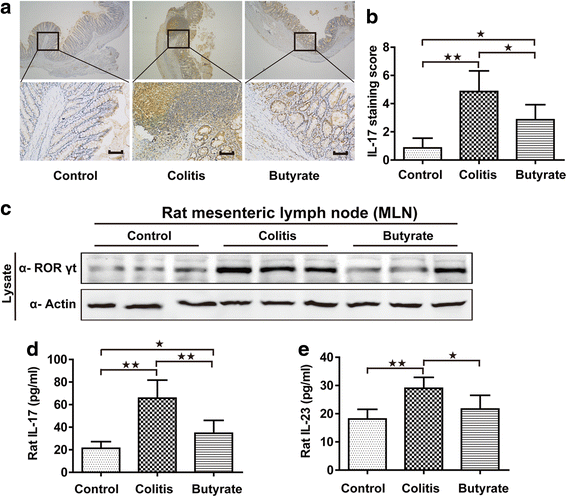
Fig. 4
Th17 analysis in rats. IL-17 immunohistochemical staining in the colon; upper and lower panel magnifications are × 40 and × 200, respectively. Scale bars, 200 μm (a). Quantified IL-17 immunohistochemical staining in colon (b). Immunoblotting for RORγt in mesenteric lymph nodes, shown are representative western blot results of three rats (c). Plasma IL-17 (d). Plasma IL-23 (e). n = 5–7. Data are the mean ± SE. n = 7. *P < 0.05; **P < 0.01
Fig. 5
Treg cell differentiation in vitro. The percentage of CD4+CD25+Foxp3+ Tregs (a). Quantified CD4+CD25+Foxp3+ Tregs (b). Culture supernatant IL-6 (c). Culture supernatant TGF-β (d). Data are the mean ± SE. n = 4. *P < 0.05; **P < 0.01. But40, 40 μM sodium butyrate. But80, 80 μM sodium butyrate
Fig. 6
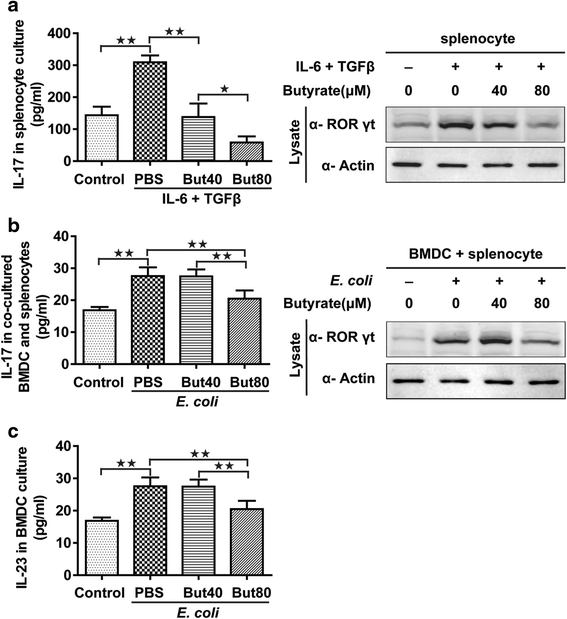
Th17 cell differentiation in vitro. IL-17 release from rat splenocytes (left) and immunoblotting for RORγt in rat splenocytes (right) in vitro (a). IL-17 release from co-cultured BMDCs and splenocytes (left) and immunoblotting for RORγt in co-cultured BMDCs and splenocytes (right) in vitro (b). IL-23 released from the BMDCs with UV-irradiated E. coli stimulation (c). Data are the mean ± SE. n = 4. *P < 0.05; **P < 0.01. But40, 40 μM sodium butyrate. But80, 80 μM sodium butyrate
Similar articles
- Kuijieling regulates the differentiation of Treg and Th17 cells to ameliorate experimental colitis in rats.
Long Y, Li S, Qin J, Xie L, Gan L, Jie F, Wu Y, Li Y, Du Q. Long Y, et al. Biomed Pharmacother. 2018 Sep;105:781-788. doi: 10.1016/j.biopha.2018.06.011. Epub 2018 Jun 15. Biomed Pharmacother. 2018. PMID: 29909346 - Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1.
Zhou L, Zhang M, Wang Y, Dorfman RG, Liu H, Yu T, Chen X, Tang D, Xu L, Yin Y, Pan Y, Zhou Q, Zhou Y, Yu C. Zhou L, et al. Inflamm Bowel Dis. 2018 Aug 16;24(9):1926-1940. doi: 10.1093/ibd/izy182. Inflamm Bowel Dis. 2018. PMID: 29796620 - Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats.
Zhang M, Qiu X, Zhang H, Yang X, Hong N, Yang Y, Chen H, Yu C. Zhang M, et al. PLoS One. 2014 Oct 2;9(10):e109146. doi: 10.1371/journal.pone.0109146. eCollection 2014. PLoS One. 2014. PMID: 25275569 Free PMC article. - Dietary resistant starch and chronic inflammatory bowel diseases.
Jacobasch G, Schmiedl D, Kruschewski M, Schmehl K. Jacobasch G, et al. Int J Colorectal Dis. 1999 Nov;14(4-5):201-11. doi: 10.1007/s003840050212. Int J Colorectal Dis. 1999. PMID: 10647628 Review. - Review article: the role of butyrate on colonic function.
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Hamer HM, et al. Aliment Pharmacol Ther. 2008 Jan 15;27(2):104-19. doi: 10.1111/j.1365-2036.2007.03562.x. Epub 2007 Oct 25. Aliment Pharmacol Ther. 2008. PMID: 17973645 Review.
Cited by
- Impaired Butyrate Induced Regulation of T Cell Surface Expression of CTLA-4 in Patients with Ulcerative Colitis.
Magnusson MK, Vidal A, Maasfeh L, Isaksson S, Malhotra R, Olsson HK, Öhman L. Magnusson MK, et al. Int J Mol Sci. 2021 Mar 17;22(6):3084. doi: 10.3390/ijms22063084. Int J Mol Sci. 2021. PMID: 33802979 Free PMC article. - Impact of COVID-19 on the Gastrointestinal Tract: A Clinical Review.
Ghazanfar H, Kandhi S, Shin D, Muthumanickam A, Gurjar H, Qureshi ZA, Shaban M, Farag M, Haider A, Budhathoki P, Bhatt T, Ghazanfar A, Jyala A, Patel H. Ghazanfar H, et al. Cureus. 2022 Mar 20;14(3):e23333. doi: 10.7759/cureus.23333. eCollection 2022 Mar. Cureus. 2022. PMID: 35464519 Free PMC article. Review. - The Insider: Impact of the Gut Microbiota on Cancer Immunity and Response to Therapies in Multiple Myeloma.
Brevi A, Cogrossi LL, Lorenzoni M, Mattorre B, Bellone M. Brevi A, et al. Front Immunol. 2022 Mar 17;13:845422. doi: 10.3389/fimmu.2022.845422. eCollection 2022. Front Immunol. 2022. PMID: 35371048 Free PMC article. Review. - Effect of Bifidobacterium on osteoclasts: TNF-α/NF-κB inflammatory signal pathway-mediated mechanism.
Wu Y, Yang Y, Wang L, Chen Y, Han X, Sun L, Chen H, Chen Q. Wu Y, et al. Front Endocrinol (Lausanne). 2023 Mar 9;14:1109296. doi: 10.3389/fendo.2023.1109296. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967748 Free PMC article. Review. - Genetics, Immunity and Nutrition Boost the Switching from NASH to HCC.
Dongiovanni P, Meroni M, Longo M, Fargion S, Fracanzani AL. Dongiovanni P, et al. Biomedicines. 2021 Oct 23;9(11):1524. doi: 10.3390/biomedicines9111524. Biomedicines. 2021. PMID: 34829753 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical