A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis - PubMed (original) (raw)
Review
A Greek Tragedy: The Growing Complexity of Alzheimer Amyloid Precursor Protein Proteolysis
Robert J Andrew et al. J Biol Chem. 2016.
Abstract
Proteolysis of the amyloid precursor protein (APP) liberates various fragments including the proposed initiator of Alzheimer disease-associated dysfunctions, amyloid-β. However, recent evidence suggests that the accepted view of APP proteolysis by the canonical α-, β-, and γ-secretases is simplistic, with the discovery of a number of novel APP secretases (including δ- and η-secretases, alternative β-secretases) and additional metabolites, some of which may also cause synaptic dysfunction. Furthermore, various proteins have been identified that interact with APP and modulate its cleavage by the secretases. Here, we give an overview of the increasingly complex picture of APP proteolysis.
Keywords: ADAM; ADAM10; Alzheimer disease; amyloid; amyloid precursor protein (APP); amyloid-beta (AB); beta-secretase 1 (BACE1); cathepsin B (CTSB); presenilin; protease; proteolysis; secretase.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
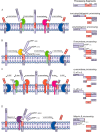
FIGURE 1.
The proteolytic processing of APP. A, the traditional model of APP proteolysis involves APP processing either in the non-amyloidogenic pathway, where sequential cleavage by α-secretase (ADAM10; pink) and the γ-secretase multisubunit complex (here shown for simplicity as a single entity; blue) liberates sAPPα and p3, or in the amyloidogenic pathway, where sequential cleavage by β-secretase (BACE1 or possibly cathepsin B; green) and γ-secretase liberates sAPPβ and Aβ. Both pathways produce AICD, which can be proteolytically degraded or translocated to the nucleus, where it has roles in transcriptional regulation. B, APP processing by the δ-secretase (AEP; yellow) causes the release of three soluble fragments of APP (sAPP1–585, sAPP1–373, and sAPP374–585). The remaining CTFδ is then further processed by β- and γ-secretases to release Aβ and AICD. C, η-secretase (suggested to be MT5-MMP; orange) releases the soluble sAPPη fragment and leaves the membrane-bound CTFη. CTFη can be further processed by α- or β-secretase to release Aη-α or Aη-β, respectively. Following α- or β-cleavage, the remaining CTF can be cleaved by γ-secretase to release p3 and AICD, or Aβ and AICD, respectively. sec, secretase. D, meprin β (purple) acts as a β-secretase producing a fragment similar to sAPPβ as well as two shorter, soluble fragments (sAPP1–380/3 and sAPP1–124). The remaining CTF can then be processed by γ-secretase to release Aβ2-X and AICD. See text for further details.
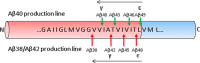
FIGURE 2.
Formation of Aβ by γ-secretase cleavage of APP CTFβ. Cleavage of APP CTFβ by γ-secretase follows a “nibbling” pattern in the direction indicated by the black arrows, where the initial (ϵ) cleavage dictates the final (γ) cleavage. The initial cleavage dictates the C-terminal length of Aβ and thus its amyloidogenic potential. N, N terminus; C, C terminus.
Similar articles
- η-Secretase processing of APP inhibits neuronal activity in the hippocampus.
Willem M, Tahirovic S, Busche MA, Ovsepian SV, Chafai M, Kootar S, Hornburg D, Evans LD, Moore S, Daria A, Hampel H, Müller V, Giudici C, Nuscher B, Wenninger-Weinzierl A, Kremmer E, Heneka MT, Thal DR, Giedraitis V, Lannfelt L, Müller U, Livesey FJ, Meissner F, Herms J, Konnerth A, Marie H, Haass C. Willem M, et al. Nature. 2015 Oct 15;526(7573):443-7. doi: 10.1038/nature14864. Epub 2015 Aug 31. Nature. 2015. PMID: 26322584 Free PMC article. - Alcadein cleavages by amyloid beta-precursor protein (APP) alpha- and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunction.
Hata S, Fujishige S, Araki Y, Kato N, Araseki M, Nishimura M, Hartmann D, Saftig P, Fahrenholz F, Taniguchi M, Urakami K, Akatsu H, Martins RN, Yamamoto K, Maeda M, Yamamoto T, Nakaya T, Gandy S, Suzuki T. Hata S, et al. J Biol Chem. 2009 Dec 25;284(52):36024-36033. doi: 10.1074/jbc.M109.057497. Epub 2009 Oct 28. J Biol Chem. 2009. PMID: 19864413 Free PMC article. - Revisiting APP secretases: an overview on the holistic effects of retinoic acid receptor stimulation in APP processing.
Vitória JJM, Trigo D, da Cruz E Silva OAB. Vitória JJM, et al. Cell Mol Life Sci. 2022 Jan 28;79(2):101. doi: 10.1007/s00018-021-04090-4. Cell Mol Life Sci. 2022. PMID: 35089425 Free PMC article. Review. - Trafficking and proteolytic processing of amyloid precursor protein and secretases in Alzheimer's disease development: An up-to-date review.
Yuksel M, Tacal O. Yuksel M, et al. Eur J Pharmacol. 2019 Aug 5;856:172415. doi: 10.1016/j.ejphar.2019.172415. Epub 2019 May 24. Eur J Pharmacol. 2019. PMID: 31132354 Review. - The Association of Amyloid-β Protein Precursor With α- and β-Secretases in Mouse Cerebral Cortex Synapses Is Altered in Early Alzheimer's Disease.
Pliássova A, Lopes JP, Lemos C, Oliveira CR, Cunha RA, Agostinho P. Pliássova A, et al. Mol Neurobiol. 2016 Oct;53(8):5710-21. doi: 10.1007/s12035-015-9491-9. Epub 2015 Oct 26. Mol Neurobiol. 2016. PMID: 26497029
Cited by
- Proteolytic shedding of the prion protein via activation of metallopeptidase ADAM10 reduces cellular binding and toxicity of amyloid-β oligomers.
Jarosz-Griffiths HH, Corbett NJ, Rowland HA, Fisher K, Jones AC, Baron J, Howell GJ, Cowley SA, Chintawar S, Cader MZ, Kellett KAB, Hooper NM. Jarosz-Griffiths HH, et al. J Biol Chem. 2019 Apr 26;294(17):7085-7097. doi: 10.1074/jbc.RA118.005364. Epub 2019 Mar 14. J Biol Chem. 2019. PMID: 30872401 Free PMC article. - The amyloid precursor protein derivative, APP96-110, is efficacious following intravenous administration after traumatic brain injury.
Plummer SL, Corrigan F, Thornton E, Woenig JA, Vink R, Cappai R, Van Den Heuvel C. Plummer SL, et al. PLoS One. 2018 Jan 10;13(1):e0190449. doi: 10.1371/journal.pone.0190449. eCollection 2018. PLoS One. 2018. PMID: 29320530 Free PMC article. - Hydrolysis of a second Asp-Pro site at the N-terminus of NOTCH3 in inherited vascular dementia.
Zhang X, Lee SJ, Wang MM. Zhang X, et al. Sci Rep. 2021 Aug 26;11(1):17246. doi: 10.1038/s41598-021-96679-9. Sci Rep. 2021. PMID: 34446744 Free PMC article. - The amyloid precursor protein and its derived fragments concomitantly contribute to the alterations of mitochondrial transport machinery in Alzheimer's disease.
Vaillant-Beuchot L, Eysert F, Duval B, Kinoshita PF, Pardossi-Piquard R, Bauer C, Eddarkaoui S, Buée L, Checler F, Chami M. Vaillant-Beuchot L, et al. Cell Death Dis. 2024 May 28;15(5):367. doi: 10.1038/s41419-024-06742-2. Cell Death Dis. 2024. PMID: 38806484 Free PMC article. - SUMO1 impact on Alzheimer disease pathology in an amyloid-depositing mouse model.
Knock E, Matsuzaki S, Takamura H, Satoh K, Rooke G, Han K, Zhang H, Staniszewski A, Katayama T, Arancio O, Fraser PE. Knock E, et al. Neurobiol Dis. 2018 Feb;110:154-165. doi: 10.1016/j.nbd.2017.11.015. Epub 2017 Dec 5. Neurobiol Dis. 2018. PMID: 29217476 Free PMC article.
References
- Rajendran L., and Annaert W. (2012) Membrane trafficking pathways in Alzheimer's disease. Traffic 13, 759–770 - PubMed
- De Strooper B., and Karran E. (2016) The cellular phase of Alzheimer's disease. Cell 164, 603–615 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- R01 AG019070/AG/NIA NIH HHS/United States
- R21 AG051230/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous