Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial - PubMed (original) (raw)
Clinical Trial
. 2016 Aug 2;316(5):500-8.
doi: 10.1001/jama.2016.10260.
Adrian F Hernandez 2, Margaret M Redfield 3, Michael M Givertz 4, Guilherme H Oliveira 5, Robert Cole 6, Douglas L Mann 7, David J Whellan 8, Michael S Kiernan 9, G Michael Felker 10, Steven E McNulty 2, Kevin J Anstrom 2, Monica R Shah 11, Eugene Braunwald 4, Thomas P Cappola 1; NHLBI Heart Failure Clinical Research Network
Collaborators, Affiliations
- PMID: 27483064
- PMCID: PMC5021525
- DOI: 10.1001/jama.2016.10260
Clinical Trial
Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial
Kenneth B Margulies et al. JAMA. 2016.
Abstract
Importance: Abnormal cardiac metabolism contributes to the pathophysiology of advanced heart failure with reduced left ventricular ejection fraction (LVEF). Glucagon-like peptide 1 (GLP-1) agonists have shown cardioprotective effects in early clinical studies of patients with advanced heart failure, irrespective of type 2 diabetes status.
Objective: To test whether therapy with a GLP-1 agonist improves clinical stability following hospitalization for acute heart failure.
Design, setting, and participants: Phase 2, double-blind, placebo-controlled randomized clinical trial of patients with established heart failure and reduced LVEF who were recently hospitalized. Patients were enrolled between August 2013 and March 2015 at 24 US sites.
Interventions: The GLP-1 agonist liraglutide (n = 154) or placebo (n = 146) via a daily subcutaneous injection; study drug was advanced to a dosage of 1.8 mg/d during the first 30 days as tolerated and continued for 180 days.
Main outcomes and measures: The primary end point was a global rank score in which all patients, regardless of treatment assignment, were ranked across 3 hierarchical tiers: time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide level from baseline to 180 days. Higher values indicate better health (stability). Exploratory secondary outcomes included primary end point components, cardiac structure and function, 6-minute walk distance, quality of life, and combined events.
Results: Among the 300 patients who were randomized (median age, 61 years [interquartile range {IQR}, 52-68 years]; 64 [21%] women; 178 [59%] with type 2 diabetes; median LVEF of 25% [IQR, 19%-33%]; median N-terminal pro-B-type natriuretic peptide level of 2049 pg/mL [IQR, 1054-4235 pg/mL]), 271 completed the study. Compared with placebo, liraglutide had no significant effect on the primary end point (mean rank of 146 for the liraglutide group vs 156 for the placebo group, P = .31). There were no significant between-group differences in the number of deaths (19 [12%] in the liraglutide group vs 16 [11%] in the placebo group; hazard ratio, 1.10 [95% CI, 0.57-2.14]; P = .78) or rehospitalizations for heart failure (63 [41%] vs 50 [34%], respectively; hazard ratio, 1.30 [95% CI, 0.89-1.88]; P = .17) or for the exploratory secondary end points. Prespecified subgroup analyses in patients with diabetes did not reveal any significant between-group differences. The number of investigator-reported hyperglycemic events was 16 (10%) in the liraglutide group vs 27 (18%) in the placebo group and hypoglycemic events were infrequent (2 [1%] vs 4 [3%], respectively).
Conclusions and relevance: Among patients recently hospitalized with heart failure and reduced LVEF, the use of liraglutide did not lead to greater posthospitalization clinical stability. These findings do not support the use of liraglutide in this clinical situation.
Trial registration: clinicaltrials.gov Identifier: NCT01800968.
Conflict of interest statement
Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Figures
Figure 1. Patient Flow Diagram for the Functional Impact of GLP-1 for Heart Failure Treatment Study
Data on patients screened for eligibility were not available. Secondary end points were analyzed with multiple imputation techniques when data were unavailable for the end point.
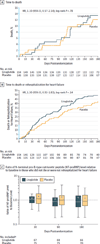
Figure 2. Components of the Primary End Point
HR indicates hazard ratio. A and B, y-axis scale in blue indicates range from 0% to 16%. The median duration of follow-up was 179 days (IQR, 157–182 days) in the liraglutide group and 178 days (IQR, 150–183 days) in the placebo group. In part C, the box plots were formed by the 25th and 75th percentiles and the line within the box is the median; the error bars indicate the 95% CIs and the data markers indicate the means. aWithout missing data.
Figure 3. Prespecified Subgroup Analysis of Patients Who Died or Experienced Rehospitalization for Heart Failure by Type 2 Diabetes Status
HR indicates hazard ratio. The median duration of follow-up was 179 days (interquartile range, 157–182 days) in the liraglutide group and 178 days (interquartile range, 150–183 days) in the placebo group.
Comment in
- Lack of Benefit for Liraglutide in Heart Failure.
Carbone S, Arena R, Abbate A. Carbone S, et al. JAMA. 2016 Dec 13;316(22):2429. doi: 10.1001/jama.2016.15387. JAMA. 2016. PMID: 27959988 No abstract available.
Similar articles
- Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial.
Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort J, Rasmussen J, Boesgaard TW, Schou M, Videbaek L, Gustafsson I, Flyvbjerg A, Wiggers H, Tarnow L. Jorsal A, et al. Eur J Heart Fail. 2017 Jan;19(1):69-77. doi: 10.1002/ejhf.657. Epub 2016 Oct 28. Eur J Heart Fail. 2017. PMID: 27790809 Clinical Trial. - Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial.
Gheorghiade M, Böhm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP; ASTRONAUT Investigators and Coordinators. Gheorghiade M, et al. JAMA. 2013 Mar 20;309(11):1125-35. doi: 10.1001/jama.2013.1954. JAMA. 2013. PMID: 23478743 Clinical Trial. - Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure.
Arturi F, Succurro E, Miceli S, Cloro C, Ruffo M, Maio R, Perticone M, Sesti G, Perticone F. Arturi F, et al. Endocrine. 2017 Sep;57(3):464-473. doi: 10.1007/s12020-016-1166-4. Epub 2016 Nov 9. Endocrine. 2017. PMID: 27830456 Clinical Trial. - Practice pearl: liraglutide and cardiovascular and renal events in type 2 diabetes.
Guthrie R. Guthrie R. Postgrad Med. 2018 Mar;130(2):154-158. doi: 10.1080/00325481.2018.1430446. Epub 2018 Jan 25. Postgrad Med. 2018. PMID: 29350569 Review. - Cardiovascular Effects of Liraglutide.
Mikhail N. Mikhail N. Curr Hypertens Rev. 2019;15(1):64-69. doi: 10.2174/1573402114666180507152620. Curr Hypertens Rev. 2019. PMID: 29737256 Review.
Cited by
- Glucagon-like peptide-1 agonists in cardiovascular diseases: a bibliometric analysis from inception to 2023.
Mahapatro A, Bozorgi A, Obulareddy SUJ, Jain SM, Reddy Korsapati R, Kumar A, Patel K, Soltani Moghadam S, Arya A, Jameel Alotaibi A, Keivanlou MH, Hassanipour S, Hasanpour M, Amini-Salehi E. Mahapatro A, et al. Ann Med Surg (Lond). 2024 Sep 25;86(11):6602-6618. doi: 10.1097/MS9.0000000000002592. eCollection 2024 Nov. Ann Med Surg (Lond). 2024. PMID: 39525800 Free PMC article. Review. - Impact of semaglutide on weight and functional outcomes among obese heart failure patients: a propensity scores matching analysis.
Balata M, Becher MU. Balata M, et al. BMC Cardiovasc Disord. 2024 Oct 26;24(1):590. doi: 10.1186/s12872-024-04275-2. BMC Cardiovasc Disord. 2024. PMID: 39462311 Free PMC article. - Changing the paradigm in heart failure: shifting from treatment to prevention.
Chang AJ, Liang Y, Girouard MP, Bhatt AS, Sandhu AT, Sauer AJ, Greene SJ, Harrington J, Go AS, Ambrosy AP. Chang AJ, et al. Heart Fail Rev. 2024 Oct 23. doi: 10.1007/s10741-024-10454-2. Online ahead of print. Heart Fail Rev. 2024. PMID: 39441333 Review. - Exploring the clinical effectiveness of glucagon-like peptide-1 receptor agonists in managing cardiovascular complications: an updated comprehensive review and future directives.
Joshi N, Qasim MZ, Kanumilli S, Shaukat F, Kumar A, Mahek F, Khalid S, Zeeshan M, Shaik MY, Nishat SM, Gandhi F, Belletieri C. Joshi N, et al. Ann Med Surg (Lond). 2024 Aug 22;86(10):5947-5956. doi: 10.1097/MS9.0000000000002494. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359798 Free PMC article. Review.
References
- Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(2):219–226. - PubMed
- Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61(2):297–306. - PubMed
- Swan JW, Anker SD, Walton C, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30(2):527–532. - PubMed
- Grigoropoulou P, Eleftheriadou I, Zoupas C, Diamanti-Kandarakis E, Tentolouris N. Incretin-based therapies for type 2 diabetes mellitus: effects on insulin resistance. Curr Diabetes Rev. 2013;9(5):412–417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HL110336/HL/NHLBI NIH HHS/United States
- U10 HL110338/HL/NHLBI NIH HHS/United States
- U10 HL110342/HL/NHLBI NIH HHS/United States
- U01 HL084861/HL/NHLBI NIH HHS/United States
- U10 HL084904/HL/NHLBI NIH HHS/United States
- U10 HL110312/HL/NHLBI NIH HHS/United States
- U10 HL110262/HL/NHLBI NIH HHS/United States
- U10 HL110337/HL/NHLBI NIH HHS/United States
- U10 HL110302/HL/NHLBI NIH HHS/United States
- U10 HL110297/HL/NHLBI NIH HHS/United States
- U10 HL110309/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical