Standardised Outcomes in Nephrology-Children and Adolescents (SONG-Kids): a protocol for establishing a core outcome set for children with chronic kidney disease - PubMed (original) (raw)
doi: 10.1186/s13063-016-1528-5.
Susan Samuel 3, Michael Zappitelli 4, Allison Dart 5, Susan Furth 6, Allison Eddy 7, Jaap Groothoff 8, Nicholas J A Webb 9, Hui-Kim Yap 10, Detlef Bockenhauer 11, Aditi Sinha 12, Stephen I Alexander 13, Stuart L Goldstein 14, Debbie S Gipson 15, Camilla S Hanson 16 13, Nicole Evangelidis 16 13, Sally Crowe 17, Tess Harris 18, Brenda R Hemmelgarn 19, Braden Manns 19, John Gill 20, Peter Tugwell 21, Wim Van Biesen 22, David C Wheeler 23, Wolfgang C Winkelmayer 24, Jonathan C Craig 16 13; SONG-Kids Investigators
Affiliations
- PMID: 27519274
- PMCID: PMC4982996
- DOI: 10.1186/s13063-016-1528-5
Standardised Outcomes in Nephrology-Children and Adolescents (SONG-Kids): a protocol for establishing a core outcome set for children with chronic kidney disease
Allison Tong et al. Trials. 2016.
Abstract
Background: Children with chronic kidney disease (CKD), requiring dialysis or kidney transplantation, have a mortality rate of up to 30-fold higher than the general aged-matched population, and severely impaired quality of life. Symptoms such as fatigue and pain are prevalent and debilitating. Children with CKD are at risk of cognitive impairment, and poorer educational, vocational, and psychosocial outcomes compared with their well peers, which have consequences through to adulthood. Treatment regimens for children with CKD are long-term, complex, and highly intrusive. While many trials have been conducted to improve outcomes in children with CKD, the outcomes measured and reported are often not relevant to patients and clinicians, and are highly variable. These problems can diminish the value of trials as a means to improve the lives of children with CKD. The Standardised Outcomes in Nephrology-Children and Adolescents (SONG-Kids) study aims to develop a core outcome set for trials in children and adolescents with any stage of CKD that is based on the shared priorities of all stakeholders.
Methods/design: SONG-Kids involves five phases: a systematic review to identify outcomes (both domains and measures) that have been reported in randomised controlled trials involving children aged up to 21 years with CKD; focus groups (using nominal group technique) with adolescent patients and caregivers of paediatric patients (all ages) to identify outcomes that are relevant and important to patients and their family and the reasons for their choices; semistructured key informant interviews with health professionals involved in the care of children with CKD to ascertain their views on establishing core outcomes in paediatric nephrology; an international three-round online Delphi survey with patients, caregivers, clinicians, researchers, policy-makers, and members from industry to develop consensus on important outcome domains; and a stakeholder workshop to review and finalise the set of core outcome domains for trials in children with CKD (including nondialysis-dependent, dialysis, and kidney transplantation).
Discussion: Establishing a core outcome set to be reported in all trials conducted in children with any stage of CKD will enhance the relevance, transparency, and impact of research to improve the lives of children and adolescents with CKD.
Keywords: Chronic kidney disease; Clinical trials; Core outcome set; Dialysis; Haemodialysis; Outcomes research; Paediatrics; Patient-centred outcomes.
Figures
Fig. 1
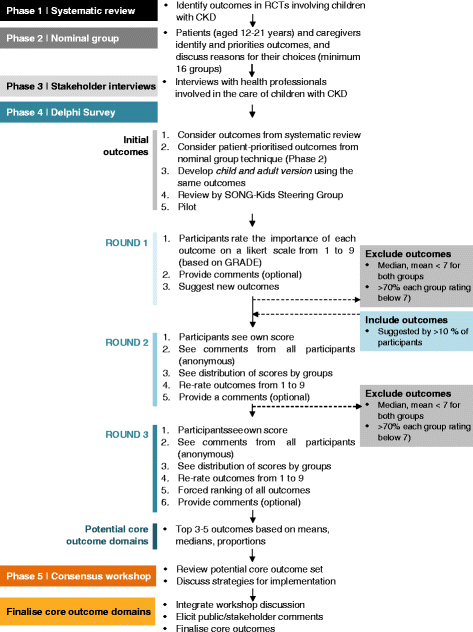
SONG-Kids process
Fig. 2
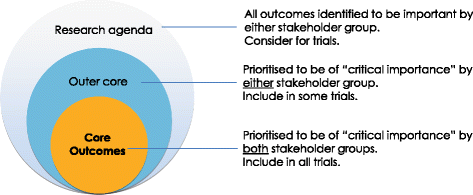
Conceptual schema of core outcomes
Similar articles
- Standardised Outcomes in Nephrology - Chronic Kidney Disease (SONG-CKD): a protocol for establishing a core outcome set for adults with chronic kidney disease who do not require kidney replacement therapy.
Evangelidis N, Sautenet B, Madero M, Tong A, Ashuntantang G, Sanabria LC, de Boer IH, Fung S, Gallego D, Levey AS, Levin A, Lorca E, Okpechi IG, Rossignol P, Sola L, Usherwood T, Wheeler DC, Cho Y, Howell M, Guha C, Scholes-Robertson N, Widders K, Gonzalez AM, Teixeira-Pinto A, Viecelli AK, Bernier-Jean A, Anumudu S, Dunn L, Wilkie M, Craig JC; SONG-CKD Investigators. Evangelidis N, et al. Trials. 2021 Sep 9;22(1):612. doi: 10.1186/s13063-021-05574-1. Trials. 2021. PMID: 34503563 Free PMC article. - Standardised outcomes in nephrology - Haemodialysis (SONG-HD): study protocol for establishing a core outcome set in haemodialysis.
Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Winkelmayer WC, van Biesen W, Crowe S, Kerr PG, Polkinghorne KR, Howard K, Pollock C, Hawley CM, Johnson DW, McDonald SP, Gallagher MP, Urquhart-Secord R, Craig JC; SONG-HD Collaboration. Tong A, et al. Trials. 2015 Aug 19;16:364. doi: 10.1186/s13063-015-0895-7. Trials. 2015. PMID: 26285819 Free PMC article. - Standardised Outcomes in Nephrology-Polycystic Kidney Disease (SONG-PKD): study protocol for establishing a core outcome set in polycystic kidney disease.
Cho Y, Sautenet B, Rangan G, Craig JC, Ong ACM, Chapman A, Ahn C, Chen D, Coolican H, Kao JT, Gansevoort R, Perrone R, Harris T, Torres V, Pei Y, Kerr PG, Ryan J, Gutman T, Howell M, Ju A, Manera KE, Teixeira-Pinto A, Hamiwka LA, Tong A. Cho Y, et al. Trials. 2017 Nov 23;18(1):560. doi: 10.1186/s13063-017-2298-4. Trials. 2017. PMID: 29169385 Free PMC article. - Developing Consensus-Based Outcome Domains for Trials in Children and Adolescents With CKD: An International Delphi Survey.
Logeman C, Guha C, Howell M, Hanson CS, Craig JC, Samuel S, Zappitelli M, Matsuda-Abedini M, Dart A, Furth S, Eddy A, Groothoff J, Yap HK, Bockenhauer D, Sinha A, Alexander SI, Goldstein SL, Gipson DS, Michael M, Walker A, Kausman J, Gaillard S, Bacchetta J, Rheault MN, Warady BA, Neu A, Christian M, McTaggart S, Liu I, Teo S, Sautenet B, Gutman T, Carter S, Teixeira-Pinto A, Tong A. Logeman C, et al. Am J Kidney Dis. 2020 Oct;76(4):533-545. doi: 10.1053/j.ajkd.2020.03.014. Epub 2020 Jul 10. Am J Kidney Dis. 2020. PMID: 32654889 - Outcome Measures in Rheumatology - Interventions for medication Adherence (OMERACT-Adherence) Core Domain Set for Trials of Interventions for Medication Adherence in Rheumatology: 5 Phase Study Protocol.
Kelly A, Tong A, Tymms K, March L, Craig JC, De Vera M, Evans V, Hassett G, Toupin-April K, van den Bemt B, Teixeira-Pinto A, Alten R, Bartlett SJ, Campbell W, Dawson T, Gill M, Hebing R, Meara A, Nieuwlaat R, Shaw Y, Singh JA, Suarez-Almazor M, Sumpton D, Wong P, Christensen R, Beaton D, de Wit M, Tugwell P; OMERACT-Adherence Group. Kelly A, et al. Trials. 2018 Mar 27;19(1):204. doi: 10.1186/s13063-018-2565-z. Trials. 2018. PMID: 29587864 Free PMC article.
Cited by
- Life outcomes after paediatric kidney transplantation: a qualitative, biographical study in long-term survivors.
Ritschl V, Stamm T, Selzer A, Boesendorfer A, Eibensteiner F, Kaltenegger L, Mosor E, Omara M, Vachuda N, Sperl L, Masel EK, Aufricht C, Boehm M. Ritschl V, et al. Arch Dis Child. 2024 Feb 19;109(3):240-246. doi: 10.1136/archdischild-2023-326432. Arch Dis Child. 2024. PMID: 38212079 Free PMC article. - The NeoPACE study: study protocol for the development of a core outcome set for neonatal palliative care.
Gallagher K, Chant K, Mancini A, Bluebond-Langner M, Marlow N. Gallagher K, et al. BMC Palliat Care. 2023 Dec 19;22(1):203. doi: 10.1186/s12904-023-01326-x. BMC Palliat Care. 2023. PMID: 38114987 Free PMC article. - Utilising low-cost, easy-to-use microscopy techniques for early peritonitis infection screening in peritoneal dialysis patients.
Buckup M, Kaneda JM, Birk AM, Glockner E, Venook R, Jain A, Sharma S, Wong C, Sutha K. Buckup M, et al. Sci Rep. 2022 Aug 18;12(1):14046. doi: 10.1038/s41598-022-18380-9. Sci Rep. 2022. PMID: 35982214 Free PMC article. - Is Preemptive Kidney Transplantation Associated With Improved Outcomes when Compared to Non-preemptive Kidney Transplantation in Children? A Systematic Review and Meta-Analysis.
Rana Magar R, Knight S, Stojanovic J, Marks SD, Lafranca JA, Turner S, Dor FJMF, Pengel LHM. Rana Magar R, et al. Transpl Int. 2022 Mar 17;35:10315. doi: 10.3389/ti.2022.10315. eCollection 2022. Transpl Int. 2022. PMID: 35368639 Free PMC article. Review. - Systematic review on outcomes used in clinical research on autosomal recessive polycystic kidney disease-are patient-centered outcomes our blind spot?
Gimpel C, Liebau MC, Schaefer F. Gimpel C, et al. Pediatr Nephrol. 2021 Dec;36(12):3841-3851. doi: 10.1007/s00467-021-05192-8. Epub 2021 Aug 12. Pediatr Nephrol. 2021. PMID: 34386850 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical