Engineered Tissue-Stent Biocomposites as Tracheal Replacements - PubMed (original) (raw)
. 2016 Sep;22(17-18):1086-97.
doi: 10.1089/ten.TEA.2016.0132.
Sumati Sundaram 2 1, Andrew V Le 1, Angela H Huang 2, Jiasheng Zhang 3, Go Hatachi 1, Arkadi Beloiartsev 1, Michael G Caty 4, Tai Yi 5, Katherine Leiby 2, Ashley Gard 2, Mehmet H Kural 1, Liqiong Gui 1, Kevin A Rocco 1, Amogh Sivarapatna 2, Elizabeth Calle 2, Allison Greaney 2, Luca Urbani 6, Panagiotis Maghsoudlou 6, Alan Burns 6 7, Paolo DeCoppi 6, Laura E Niklason 2 1
Affiliations
- PMID: 27520928
- PMCID: PMC5312617
- DOI: 10.1089/ten.TEA.2016.0132
Engineered Tissue-Stent Biocomposites as Tracheal Replacements
Liping Zhao et al. Tissue Eng Part A. 2016 Sep.
Abstract
Here we report the creation of a novel tracheal construct in the form of an engineered, acellular tissue-stent biocomposite trachea (TSBT). Allogeneic or xenogeneic smooth muscle cells are cultured on polyglycolic acid polymer-metal stent scaffold leading to the formation of a tissue comprising cells, their deposited collagenous matrix, and the stent material. Thorough decellularization then produces a final acellular tubular construct. Engineered TSBTs were tested as end-to-end tracheal replacements in 11 rats and 3 nonhuman primates. Over a period of 8 weeks, no instances of airway perforation, infection, stent migration, or erosion were observed. Histological analyses reveal that the patent implants remodel adaptively with native host cells, including formation of connective tissue in the tracheal wall and formation of a confluent, columnar epithelium in the graft lumen, although some instances of airway stenosis were observed. Overall, TSBTs resisted collapse and compression that often limit the function of other decellularized tracheal replacements, and additionally do not require any cells from the intended recipient. Such engineered TSBTs represent a model for future efforts in tracheal regeneration.
Conflict of interest statement
L.E.N. is a founder and shareholder in Humacyte, Inc., which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic SMCs for vascular surgery. L.E.N.'s spouse has equity in Humacyte, and L.E.N. serves on Humacyte's Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte and that produce royalties for L.E.N. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale. Humacyte did not fund this study and did not influence the conduct, description, or interpretation of the findings in this report.
Figures
**FIG. 1.
Creation of the engineered TSBT. (A) Clinical-grade metal nitinol stent, 6 mm diameter. (B) Stent–polymer (polyglycolic acid) composite scaffold. (C) Bioreactor culture of smooth muscle cells on stent–polymer scaffold. (D) Engineered cellular tissue–stent composite at the end of 8 weeks. (E) Final product for tracheal replacement—4-mm diameter decellularized engineered TSBT. (F) Gross picture of a 16-mm diameter TSBT, of a size suitable for clinical airway repair. TSBT, tissue–stent biocomposite trachea.
**FIG. 2.
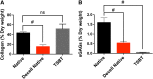
Biochemical characterization of engineered TSBTs. (A) Collagen quantified by hydroxyproline assay for native rat, decellularized native rat trachea, and TSBTs. (B) sGAGs quantified by Blyscan assay for native rat trachea, decellularized native rat trachea, and TSBT. ns, not significant; #p < 0.01 (n = 4 for native rat trachea, n = 3 for decellularized native rat trachea, n = 3 for TSBT). sGAGs, sulfated glycosaminoglycans.
**FIG. 3.
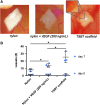
Angiogenic testing of TSBTs: chorioallantoic membrane assay. (A) Representative images of negative control (nylon membrane), nylon loaded with VEGF 200 ng/mL and engineered matrix scaffold TSBT placed in ovo at the end of 7 days of incubation. (B) Number of vessels attracted toward the matrices at days 0 and 7 postimplantation, significant differences between all groups at day 7. *p < 0.05 (day 0: n = 4 for nylon, n = 4 for nylon + VEGF, n = 3 for TSBT scaffold; day 7: n = 3 for nylon, n = 4 for nylon + VEGF, n = 3 for TSBT scaffold). VEGF, vascular endothelial growth factor.
**FIG. 4.
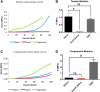
Mechanical characterization of 4-mm diameter TSBTs and rat tracheas: (A, C) Representative stress–strain curves for tensile (A) and compressive (C) loads comparing TSBT (green curve) with native (blue) and decellularized native (red) rat tracheas. Tensile and compressive moduli for TSBT and rat trachea are shown in (B) and (D), respectively. Moduli computed from slope of curves beyond 60% strain. For tensile and compression studies, n = 3 for all samples. # denotes p < 0.05.
**FIG. 5.
In vivo testing of TSBT in a rat model. (A) End-to-end implantation of a 4-mm TSBT as a tracheal replacement. (B) Explant at 8 weeks showing metal stents and remodeled tissue with new angiogenic ingrowth. (C) Luminal view of explant at 8 weeks showing a patent graft.
**FIG. 6.
Remodeling of TSBT in a rat model over 8 weeks. H&E staining of a preimplant TSBT indicating acellular nature of graft (A, D). Remodeled with cellular infiltration throughout at weeks 2 and 8 postimplant (B, C). Arrowheads in (B, C) indicate luminal surface of TSBTs. Luminal cell repopulation at 2 weeks shows cuboidal cells (E, enlarged solid red box from B) and at 8 weeks shows ciliated cells (F, enlarged solid blue box from C). Repopulation of the TSBT walls shows areas that appear to be vascularized developed at 2 weeks (G, enlarged dotted red box from B), and at 8 weeks (H, enlarged dotted blue box from C). Arrowheads in (G, H) indicate microvessels. Asterisk (*) indicates location of metal stent struts. Scale bars: (A), 500 μm; (B–D), 100 μm; (E, F), 25 μm; (G, H), 100 μm. H&E, hematoxylin and eosin.
**FIG. 7.
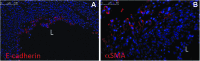
Immunofluorescence of remodeled TSBT in a rat model at 1 week. Immunofluoresence staining for (A) E-cadherin (red), a pan-epithelial marker and (B) Alpha smooth muscle actin (red), a marker for smooth muscle cells. Scale bar: (A), 100 μm; (B), 50 μm. L, lumen of TSBT.
**FIG. 8.
In vivo testing of TSBT in a primate model (A) Remodeling over 7 weeks. Low power H&E staining of TSBT in a nonhuman primate showing cross section of TSBT wall at (B) 2 weeks and (C) 7 weeks. Asterisk (*) indicate stent struts, arrowheads point to luminal surface. H&E of TSBT shows a simple cuboidal epithelium at (D) 2 weeks and multilayer pseudostratified columnar luminal cells at 7 weeks in primate explants (E). Scale bars: (B, C), 100 μm; (D, E), 20 μm.
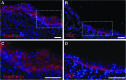
**FIG. 9.
Immunofluorescence of remodeled TSBT in a primate model at 7 weeks. Immunofluoresence staining for (A, C) E-cadherin (red), a pan-epithelial marker and for (B, D) cytokeratin5 (red), a marker for basal-like cells. CK5 staining observed at the base of the luminal cell layer. DAPI-stained nuclei are blue. (C) is enlarged from dotted area in (A); (D) is enlarged from dotted area in (B). Scale bars: (A–D) 75 μm.
**FIG. 10.
Microvessels formed in remodeled TSBT at 7 weeks in a primate model. (A) αSMA (red) positive and (B) vWF (red) positive cells lining microvascular structures of the remodeled TSBT. Nuclei in (A, B) are blue. (C) H&E staining of remodeled wall of TSBT from a primate showing vascular structures. Scale bars: (A–C) 75 μm. αSMA, alpha smooth muscle actin; vWF, von Willebrand factor.
Similar articles
- Prenatal tracheal reconstruction with a hybrid amniotic mesenchymal stem cells-engineered construct derived from decellularized airway.
Gray FL, Turner CG, Ahmed A, Calvert CE, Zurakowski D, Fauza DO. Gray FL, et al. J Pediatr Surg. 2012 Jun;47(6):1072-9. doi: 10.1016/j.jpedsurg.2012.03.006. J Pediatr Surg. 2012. PMID: 22703772 - Trachea Engineering Using a Centrifugation Method and Mouse-Induced Pluripotent Stem Cells.
Zhou Q, Ye X, Ran Q, Kitahara A, Matsumoto Y, Moriyama M, Ajioka Y, Saijo Y. Zhou Q, et al. Tissue Eng Part C Methods. 2018 Sep;24(9):524-533. doi: 10.1089/ten.TEC.2018.0115. Tissue Eng Part C Methods. 2018. PMID: 30101671 - PLGA-PTMC-Cultured Bone Mesenchymal Stem Cell Scaffold Enhances Cartilage Regeneration in Tissue-Engineered Tracheal Transplantation.
Yan B, Zhang Z, Wang X, Ni Y, Liu Y, Liu T, Wang W, Xing H, Sun Y, Wang J, Li XF. Yan B, et al. Artif Organs. 2017 May;41(5):461-469. doi: 10.1111/aor.12805. Epub 2016 Dec 7. Artif Organs. 2017. PMID: 27925229 - Biomedical grafts for tracheal tissue repairing and regeneration "Tracheal tissue engineering: an overview".
Dhasmana A, Singh A, Rawal S. Dhasmana A, et al. J Tissue Eng Regen Med. 2020 May;14(5):653-672. doi: 10.1002/term.3019. Epub 2020 Mar 19. J Tissue Eng Regen Med. 2020. PMID: 32064791 Review. - Trachea transplantation: from laboratory to patient.
Crowley C, Birchall M, Seifalian AM. Crowley C, et al. J Tissue Eng Regen Med. 2015 Apr;9(4):357-67. doi: 10.1002/term.1847. J Tissue Eng Regen Med. 2015. PMID: 26052583 Review.
Cited by
- Long-Term Survival and Regeneration Following Transplantation of 3D-Printed Biodegradable PCL Tracheal Grafts in Large-Scale Porcine Models.
Shai SE, Lai YL, Hung YW, Hsieh CW, Su KC, Wang CH, Chao TH, Chiu YT, Wu CC, Hung SC. Shai SE, et al. Bioengineering (Basel). 2024 Aug 14;11(8):832. doi: 10.3390/bioengineering11080832. Bioengineering (Basel). 2024. PMID: 39199790 Free PMC article. - Vascular adaptation in the presence of external support - A modeling study.
Ramachandra AB, Latorre M, Szafron JM, Marsden AL, Humphrey JD. Ramachandra AB, et al. J Mech Behav Biomed Mater. 2020 Oct;110:103943. doi: 10.1016/j.jmbbm.2020.103943. Epub 2020 Jun 25. J Mech Behav Biomed Mater. 2020. PMID: 32957235 Free PMC article. - Cartilaginous Extracellular Matrix Enriched with Human Gingival Mesenchymal Stem Cells Derived "Matrix Bound Extracellular Vesicles" Enabled Functional Reconstruction of Tracheal Defect.
Zeng T, Yuan P, Liang L, Zhang X, Zhang H, Wu W. Zeng T, et al. Adv Sci (Weinh). 2022 Jan;9(2):e2102735. doi: 10.1002/advs.202102735. Epub 2021 Nov 28. Adv Sci (Weinh). 2022. PMID: 34841733 Free PMC article. - The Growing Medical Need for Tracheal Replacement: Reconstructive Strategies Should Overcome Their Limits.
Adamo D, Galaverni G, Genna VG, Lococo F, Pellegrini G. Adamo D, et al. Front Bioeng Biotechnol. 2022 May 9;10:846632. doi: 10.3389/fbioe.2022.846632. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646864 Free PMC article. Review. - The History of Engineered Tracheal Replacements: Interpreting the Past and Guiding the Future.
Greaney AM, Niklason LE. Greaney AM, et al. Tissue Eng Part B Rev. 2021 Aug;27(4):341-352. doi: 10.1089/ten.TEB.2020.0238. Epub 2020 Nov 9. Tissue Eng Part B Rev. 2021. PMID: 33045942 Free PMC article.
References
- Del Gaudio C., Baiguera S., Ajalloueian F., Bianco A., and Macchiarini P. Are synthetic scaffolds suitable for the development of clinical tissue-engineered tubular organs? J Biomed Mater Res A 102, 2427, 2014 - PubMed
- Rich J.T., and Gullane P.J. Current concepts in tracheal reconstruction. Curr Opin Otolaryngol Head Neck Surg 20, 246, 2012 - PubMed
- Jungebluth P., Alici E., Baiguera S., Le Blanc K., Blomberg P., Bozóky B., Crowley C., Einarsson O., Grinnemo K.-H., Gudbjartsson T., Le Guyader S., Henriksson G., Hermanson O., Juto J.E., Leidner B., Lilja T., Liska J., Luedde T., Lundin V., Moll G., Nilsson B., Roderburg C., Strömblad S., Sutlu T., Teixeira A.I., Watz E., Seifalian A., and Macchiarini P. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 378, 1997, 2011 - PubMed
- Delaere P., Vranckx J., Verleden G., De Leyn P., Van Raemdonck D., and Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 362, 138, 2010 - PubMed
- Delaere P.R., Vranckx J.J., Den Hondt M., and Leuven Tracheal Transplant Group. Tracheal allograft after withdrawal of immunosuppressive therapy. N Engl J Med 370, 1568, 2014 - PubMed
MeSH terms
Grants and funding
- T32 GM007205/GM/NIGMS NIH HHS/United States
- T32 GM086287/GM/NIGMS NIH HHS/United States
- U01 HL111016/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources