An exploratory evaluation of Take Control: A novel computer-delivered behavioral platform for placebo-controlled pharmacotherapy trials for alcohol use disorder - PubMed (original) (raw)
Randomized Controlled Trial
An exploratory evaluation of Take Control: A novel computer-delivered behavioral platform for placebo-controlled pharmacotherapy trials for alcohol use disorder
Eric G Devine et al. Contemp Clin Trials. 2016 Sep.
Abstract
Placebo-controlled pharmacotherapy trials for alcohol use disorder (AUD) require an active behavioral platform to avoid putting participants at risk for untreated AUD and to better assess the effectiveness of the medication. Therapist-delivered platforms (TDP) can be costly and present a risk to study design because of the variability in therapist fidelity. Take Control is a novel computer-delivered behavioral platform developed for use in pharmacotherapy trials sponsored by the National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG). This behavioral platform was developed with the goal of reducing trial implementation costs and limiting potential bias introduced by therapists providing TDP. This exploratory study is the first to compare Take Control with TDP on measures related to placebo response rate, medication adherence, and participant retention. Data were drawn from the placebo arms of four multisite, double-blind, randomized controlled trials (RCT) for AUD conducted by NCIG from 2007 to 2015. Data were compared from subjects receiving TDP (n=156) in two RCTs and Take Control (n=155) in another two RCTs. Placebo response rate, as represented by weekly percentage of heavy drinking days, was similar between groups. Subjects who received Take Control had a higher rate of medication adherence than those who received TDP. Subject retention was not significantly different between groups. The findings suggest that Take Control is comparable to TDP on measures of retention, medication adherence, and placebo response. Additional research is needed to evaluate Take Control directly against TDPs in a randomized trial.
Keywords: Alcohol use disorder; Behavioral platform; Computer-based intervention; Randomized controlled trial; Take control.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
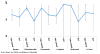
Figure 1
Placebo Response Rate (Percent Heavy Drinking Days outcome) - Take Control vs Therapist Delivered Platform
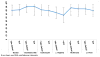
Figure 2
Medication Adherence Rate - Take Control vs vs Therapist-Delivered Platform
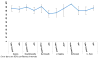
Figure 3
Prevalence of Complete Drinking Data - Take Control vsvs Therapist-Delivered Platform
Figure 4
Visit Participation Rate - Take Control vs Therapist-Delivered Platform
Similar articles
- Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study.
Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Salloum IM, et al. Arch Gen Psychiatry. 2005 Jan;62(1):37-45. doi: 10.1001/archpsyc.62.1.37. Arch Gen Psychiatry. 2005. PMID: 15630071 Clinical Trial. - Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study.
Mann K, Lemenager T, Hoffmann S, Reinhard I, Hermann D, Batra A, Berner M, Wodarz N, Heinz A, Smolka MN, Zimmermann US, Wellek S, Kiefer F, Anton RF; PREDICT Study Team. Mann K, et al. Addict Biol. 2013 Nov;18(6):937-46. doi: 10.1111/adb.12012. Epub 2012 Dec 12. Addict Biol. 2013. PMID: 23231446 Clinical Trial. - Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial.
Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A; COMBINE Study Research Group. Anton RF, et al. JAMA. 2006 May 3;295(17):2003-17. doi: 10.1001/jama.295.17.2003. JAMA. 2006. PMID: 16670409 Clinical Trial. - Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].
LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. LeBlanc EL, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review. - Control group bias in randomized atypical antipsychotic medication trials for schizophrenia.
Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Woods SW, et al. Arch Gen Psychiatry. 2005 Sep;62(9):961-70. doi: 10.1001/archpsyc.62.9.961. Arch Gen Psychiatry. 2005. PMID: 16143728 Review.
Cited by
- Treatment of Alcohol Use Disorder: Behavioral and Pharmacologic Therapies.
Tareen K, Clifton EG, Perumalswami P, Mellinger JL, Winder GS. Tareen K, et al. Clin Liver Dis. 2024 Nov;28(4):761-778. doi: 10.1016/j.cld.2024.06.011. Clin Liver Dis. 2024. PMID: 39362720 Review. - Effects of Internet-Based Cognitive Behavioral Therapy for Harmful Alcohol Use and Alcohol Dependence as Self-help or With Therapist Guidance: Three-Armed Randomized Trial.
Johansson M, Berman AH, Sinadinovic K, Lindner P, Hermansson U, Andréasson S. Johansson M, et al. J Med Internet Res. 2021 Nov 24;23(11):e29666. doi: 10.2196/29666. J Med Internet Res. 2021. PMID: 34821563 Free PMC article. Clinical Trial. - Results of a randomized, double-blind, placebo-controlled trial of lorcaserin in cocaine use disorder.
McCann DJ, Chen HH, Devine EG, Gyaw S, Ramey T. McCann DJ, et al. Drug Alcohol Depend. 2024 Feb 1;255:111063. doi: 10.1016/j.drugalcdep.2023.111063. Epub 2023 Dec 17. Drug Alcohol Depend. 2024. PMID: 38163425 Free PMC article. Clinical Trial. - State-of-the-art behavioral and pharmacological treatments for alcohol use disorder.
Ray LA PhD, Bujarski S PhD, Grodin E PhD, Hartwell E PhD, Green R MA, Venegas A BS, Lim AC MA, Gillis A MD, Miotto K MD. Ray LA PhD, et al. Am J Drug Alcohol Abuse. 2019;45(2):124-140. doi: 10.1080/00952990.2018.1528265. Epub 2018 Oct 29. Am J Drug Alcohol Abuse. 2019. PMID: 30373394 Free PMC article. Review. - Advances in the science and treatment of alcohol use disorder.
Witkiewitz K, Litten RZ, Leggio L. Witkiewitz K, et al. Sci Adv. 2019 Sep 25;5(9):eaax4043. doi: 10.1126/sciadv.aax4043. eCollection 2019 Sep. Sci Adv. 2019. PMID: 31579824 Free PMC article. Review.
References
- Amdur RJ, Biddle CJ. The placebo controlled clinical trial. In: Amdur RJ, Bankert EA, editors. Institutional Review Board: Management and Function. Jones and Bartlett; Sudbury, MA: 2006. pp. 441–449.
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States. JAMA. 2000;291:1238–1245. - PubMed
- Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97:265–277. - PubMed
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. [accessed 4.11.2016];ICH harmonised tripartite guideline, choice of control group and related issues in clinical trials E10. 2002 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources