Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy - PubMed (original) (raw)
. 2016 Nov 3;19(5):663-671.
doi: 10.1016/j.stem.2016.07.019. Epub 2016 Aug 11.
Zhifei Luo 2, Jianxiong Zeng 3, Weiqiang Chen 3, Suan-Sin Foo 3, Shin-Ae Lee 3, Jianning Ge 3, Su Wang 4, Steven A Goldman 4, Berislav V Zlokovic 2, Zhen Zhao 5, Jae U Jung 6
Affiliations
- PMID: 27524440
- PMCID: PMC5144538
- DOI: 10.1016/j.stem.2016.07.019
Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy
Qiming Liang et al. Cell Stem Cell. 2016.
Abstract
The current widespread outbreak of Zika virus (ZIKV) infection has been linked to severe clinical birth defects, particularly microcephaly, warranting urgent study of the molecular mechanisms underlying ZIKV pathogenesis. Akt-mTOR signaling is one of the key cellular pathways essential for brain development and autophagy regulation. Here, we show that ZIKV infection of human fetal neural stem cells (fNSCs) causes inhibition of the Akt-mTOR pathway, leading to defective neurogenesis and aberrant activation of autophagy. By screening the three structural proteins and seven nonstructural proteins present in ZIKV, we found that two, NS4A and NS4B, cooperatively suppress the Akt-mTOR pathway and lead to cellular dysregulation. Corresponding proteins from the closely related dengue virus do not have the same effect on neurogenesis. Thus, our study highlights ZIKV NS4A and NS4B as candidate determinants of viral pathogenesis and identifies a mechanism of action for their effects, suggesting potential targets for anti-ZIKV therapeutic intervention.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
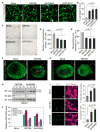
Figure 1. ZIKV Infection Impairs Neurosphere Formation and Elevates Autophagy in fNSCs
(A) Representative images from Live/Dead cell viability assay in cultured fNSCs at 5 dpi with three strains of ZIKV (MR766, IbH30656, or H/PF/2013) or mock treatment. Images were taken using live cell imaging. Scale bar, 20 μm. (B) Quantification of percentage of cell death as described in (A). Mean ± SEM; p < 0.05 by one-way ANOVA. (C) Representative images showing neurosphere formation at 3 dpi with three strains of ZIKV or mock treatment. Scale bar, 100 μm. (D and E) Quantification of number of neurospheres formed per 1 × 105 fNSCs (D) and neurosphere size by diameter measurement (E) in conditions as in (C). Mean ± SEM; p < 0.05 by one-way ANOVA. (F and G) Representative confocal images showing 3D reconstruction of neurospheres at 7 dpi with MR766 or mock treatment. Nestin and SOX2 were used as fNSC-specific markers; ZIKV was immunostained against its E protein in (F); apoptotic cell death was marked by TUNEL staining in (G). Scale bar, 50 μm. (H) ZIKV infection induces autophagy in fNSCs. fNSCs were infected with ZIKV and LC3 processing was examined by immunoblot at indicated time points. (I) fNSCs infected with ZIKV at MOI 0.1 were fixed and stained with indicated antibodies and LC3 puncta were counted. Mean ± SEM; p < 0.05 by one-way ANOVA. (J) Autophagy is required for the efficient replication of ZIKV. fNSCs were infected with ZIKV MR766 at MOI 0.5, and the medium was changed with indicated drugs (rapamycin 50 nM, 3-MA 2 μM, chloroquine 5 μM) at 1 hpi. The mRNA levels of ZIKV were measured by RT-qPCR at 10 hpi. See also Figures S1 and S2.
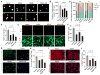
Figure 2. ZIKV NS4A and NS4B Impair Neurogenesis of fNSCs
(A) Representative images of neurospheres formed at 7 dpi from fNSCs transduced with lentiviruses expressing ZIKV NS4A, NS4B, NS4A-NS4B, or vector alone. Neurospheres were stained with fNSC markers Nestin and SOX2; upper panels represent primary neurospheres (P0), lower panels represent secondary neurospheres after passage (P1). Scale bar, 50 μm. (B) Quantification of number of neurospheres formed per 1 × 105 fNSCs transduced with lentiviruses as indicated. Mean ± SEM; p < 0.05 by one-way ANOVA. (C) Percentage bar graph showing the distribution of neurospheres at different size ranges as described in (A). (D) BrdU incorporation into fNSC-based flow cytometry analysis at 5 days after transduction with different lentiviruses as indicated. Mean ± SEM; p < 0.05 by one-way ANOVA. (E and F) Representative confocal images of Nestin and Ki-67 double staining (E) and the quantification of proliferating Nestin+ and Ki-67+ double positive fNSCs (F) at 5 days after transduction with different lentiviruses as indicated. Mean ± SEM; p < 0.05 by one-way ANOVA in (F). (G and H) Representative confocal images of β3-tubulin immunostaining on fNSCs transduced with different lentiviruses as indicated followed by 10 days of differentiation (G) and quantification (percentage) of β3-tubulin-positive neurons differentiated from fNSCs (H). Dapi: nucleus staining. Mean ± SEM; p < 0.05 by one-way ANOVA in (H). (I and J) Representative confocal images of GFAP immunostaining and nucleus staining with Dapi (I) and the quantification (percentage) of GFAP-positive astrocytes differentiated from fNSCs (J), 10 days after transduction with different lentiviruses as indicated. Mean ± SEM; p < 0.05 by one-way ANOVA in (J). See also Figure S3.
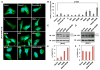
Figure 3. ZIKV NS4A and NS4B Induce Autophagy
(A and B) Screening of ZIKV proteins for autophagy induction. HeLa-GFP-LC3 cells transiently expressed each ZIKV protein as indicated by lentivirus infection. The levels of GFP-LC3 puncta were measured and quantified at 2 dpi. Mean ± SEM; p < 0.05 by one-way ANOVA in (B). (C–F) LC3 processing from fNSCs or HeLa cells stably expressing vector, NS4A, NS4B, or NS4A-NS4B was measured by immunoblot with indicated antibodies. The levels of LC3-II/LC3-I were quantified by band intensity with Image Lab software (BioRad). See also Figure S4.
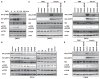
Figure 4. ZIKV NS4A and NS4B Inhibit the Akt-mTOR Signaling Pathway
(A) ZIKV replication inhibits Akt-mTOR signaling. fNSCs were infected with ZIKV strain MR766 at MOI 0.5. Cell lysates were harvested at various time points and subjected to immunoblot with indicated antibodies. (B) The levels of Akt and mTOR activities of HeLa cells expressing each ZIKV gene were measured with indicated antibodies. NS3H, NS3 helicase domain; NS3S, NS3 serine protease domain. (C–E) fNSCs or HeLa cells stably expressing vector, NS4A, NS4B, or NS4A-NS4B were stimulated with serum (20%) or insulin (2 μg/mL) after 8 hr starvation. Cell lysates were harvested and subjected to immunoblot with indicated antibodies. See also Figure S4.
Similar articles
- Interplay Between Zika Virus-Induced Autophagy and Neural Stem Cell Fate Determination.
Bindu, Pandey HS, Seth P. Bindu, et al. Mol Neurobiol. 2024 Dec;61(12):9927-9944. doi: 10.1007/s12035-023-03704-1. Epub 2023 Nov 1. Mol Neurobiol. 2024. PMID: 37910284 - Suggested mechanisms for Zika virus causing microcephaly: what do the genomes tell us?
Jun SR, Wassenaar TM, Wanchai V, Patumcharoenpol P, Nookaew I, Ussery DW. Jun SR, et al. BMC Bioinformatics. 2017 Dec 28;18(Suppl 14):471. doi: 10.1186/s12859-017-1894-3. BMC Bioinformatics. 2017. PMID: 29297281 Free PMC article. - Mechanistic Target of Rapamycin Signaling Activation Antagonizes Autophagy To Facilitate Zika Virus Replication.
Sahoo BR, Pattnaik A, Annamalai AS, Franco R, Pattnaik AK. Sahoo BR, et al. J Virol. 2020 Oct 27;94(22):e01575-20. doi: 10.1128/JVI.01575-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32878890 Free PMC article. - Autophagy in Zika Virus Infection: A Possible Therapeutic Target to Counteract Viral Replication.
Gratton R, Agrelli A, Tricarico PM, Brandão L, Crovella S. Gratton R, et al. Int J Mol Sci. 2019 Feb 28;20(5):1048. doi: 10.3390/ijms20051048. Int J Mol Sci. 2019. PMID: 30823365 Free PMC article. Review. - The impact of Zika virus in the brain.
Russo FB, Beltrão-Braga PCB. Russo FB, et al. Biochem Biophys Res Commun. 2017 Oct 28;492(4):603-607. doi: 10.1016/j.bbrc.2017.01.074. Epub 2017 Jan 17. Biochem Biophys Res Commun. 2017. PMID: 28108286 Review.
Cited by
- Functional Mapping of AGO-Associated Zika Virus-Derived Small Interfering RNAs in Neural Stem Cells.
Zeng J, Luo Z, Dong S, Xie X, Liang X, Yan Y, Liang Q, Zhao Z. Zeng J, et al. Front Cell Infect Microbiol. 2021 Feb 25;11:628887. doi: 10.3389/fcimb.2021.628887. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33718276 Free PMC article. - Zika Virus Infection Downregulates Connexin 43, Disrupts the Cardiomyocyte Gap Junctions and Induces Heart Diseases in A129 Mice.
Li S, Armstrong N, Zhao H, Cruz-Cosme R, Yang H, Zhong C, Fu W, Wang W, Yang D, Xia N, Cheng T, Tang Q. Li S, et al. J Virol. 2022 Nov 9;96(21):e0137322. doi: 10.1128/jvi.01373-22. Epub 2022 Oct 13. J Virol. 2022. PMID: 36226984 Free PMC article. - The Influence of Metabolism on Immune Response: A Journey to Understand Immunometabolism in the Context of Viral Infection.
El Safadi D, Paulo-Ramos A, Hoareau M, Roche M, Krejbich-Trotot P, Viranaicken W, Lebeau G. El Safadi D, et al. Viruses. 2023 Dec 9;15(12):2399. doi: 10.3390/v15122399. Viruses. 2023. PMID: 38140640 Free PMC article. Review. - PI3K/Akt/mTOR pathway: a potential target for anti-SARS-CoV-2 therapy.
Fattahi S, Khalifehzadeh-Esfahani Z, Mohammad-Rezaei M, Mafi S, Jafarinia M. Fattahi S, et al. Immunol Res. 2022 Jun;70(3):269-275. doi: 10.1007/s12026-022-09268-x. Epub 2022 Feb 2. Immunol Res. 2022. PMID: 35107743 Free PMC article. Review. - Molecular signatures associated with prostate cancer cell line (PC-3) exposure to inactivated Zika virus.
Delafiori J, Lima EO, Dabaja MZ, Dias-Audibert FL, de Oliveira DN, Melo CFOR, Morishita KN, Sales GM, Ruiz ALTG, da Silva GG, Lancellotti M, Catharino RR. Delafiori J, et al. Sci Rep. 2019 Oct 25;9(1):15351. doi: 10.1038/s41598-019-51954-8. Sci Rep. 2019. PMID: 31653965 Free PMC article.
References
- Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendonça MCL, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–660. - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL110609/HL/NHLBI NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R01 AI116585/AI/NIAID NIH HHS/United States
- R01 MH104701/MH/NIMH NIH HHS/United States
- R01 NS075345/NS/NINDS NIH HHS/United States
- R01 MH099578/MH/NIMH NIH HHS/United States
- R01 NS090904/NS/NINDS NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- P01 CA180779/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R35 CA200422/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous