Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1136/bmj.i4205.
Anna-Birgitte Aga 2, Inge Christoffer Olsen 2, Siri Lillegraven 2, Hilde B Hammer 2, Till Uhlig 2, Hallvard Fremstad 3, Tor Magne Madland 4, Åse Stavland Lexberg 5, Hilde Haukeland 6, Erik Rødevand 7, Christian Høili 8, Hilde Stray 9, Anne Noraas 10, Inger Johanne Widding Hansen 11, Gunnstein Bakland 12, Lena Bugge Nordberg 2, Désirée van der Heijde 13, Tore K Kvien 2
Affiliations
- PMID: 27530741
- PMCID: PMC4986519
- DOI: 10.1136/bmj.i4205
Randomized Controlled Trial
Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial
Espen A Haavardsholm et al. BMJ. 2016.
Abstract
Objective: To determine whether a treatment strategy based on structured ultrasound assessment would lead to improved outcomes in rheumatoid arthritis, compared with a conventional strategy.
Design: Multicentre, open label, two arm, parallel group, randomised controlled strategy trial.
Setting: Ten rheumatology departments and one specialist centre in Norway, from September 2010 to September 2015.
Participants: 238 patients were recruited between September 2010 and April 2013, of which 230 (141 (61%) female) received the allocated intervention and were analysed for the primary outcome. The main inclusion criteria were age 18-75 years, fulfilment of the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis, disease modifying anti-rheumatic drug naivety with indication for disease modifying drug therapy, and time from first patient reported swollen joint less than two years. Patients with abnormal kidney or liver function or major comorbidities were excluded.
Interventions: 122 patients were randomised to an ultrasound tight control strategy targeting clinical and imaging remission, and 116 patients were randomised to a conventional tight control strategy targeting clinical remission. Patients in both arms were treated according to the same disease modifying anti-rheumatic drug escalation strategy, with 13 visits over two years.
Main outcome measures: The primary endpoint was the proportion of patients with a combination between 16 and 24 months of clinical remission, no swollen joints, and non-progression of radiographic joint damage. Secondary outcomes included measures of disease activity, radiographic progression, functioning, quality of life, and adverse events. All participants who attended at least one follow-up visit were included in the full analysis set.
Results: 26 (22%) of the 118 analysed patients in the ultrasound tight control arm and 21 (19%) of the 112 analysed patients in the clinical tight control arm reached the primary endpoint (mean difference 3.3%, 95% confidence interval -7.1% to 13.7%). Secondary endpoints (disease activity, physical function, and joint damage) were similar between the two groups. Six (5%) patients in the ultrasound tight control arm and seven (6%) patients in the conventional arm had serious adverse events.
Conclusions: The systematic use of ultrasound in the follow-up of patients with early rheumatoid arthritis treated according to current recommendations is not justified on the basis of the ARCTIC results. The findings highlight the need for randomised trials assessing the clinical application of medical technology.Trial registration Clinical trials NCT01205854.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare: EAH has received research funding from Pfizer, UCB, Roche, MSD, and AbbVie for the submitted work, honorariums as a speaker from Pfizer, UCB, Roche, and AbbVie, and honorariums for development of educational material from Pfizer and has sat on advisory boards for Pfizer; ABA has sat on advisory boards for UCB, AbbVie, and Pfizer and received honorariums for development of educational material for UCB; HBH has received honorariums as a speaker from AbbVie, Bristol-Myers Squibb, Roche, UCB Pharma, and Pfizer; HH has sat on advisory boards for UCB and AbbVie; GB has received honorariums as a speaker from AbbVie and has sat on advisory boards for Pfizer; DvdH has received consultancy honorariums from AbbVie, Amgen, Astellas, AstraZeneca, Bristol-Myers Squibb, Celgene, Daiichi, Eli Lilly, Galapagos, Merck, Novartis, Pfizer, Roche, Sanofi Aventis, Janssen, and UCB and is owner of Imaging Rheumatology; TKK has received consultancy honorariums from AbbVie, Bristol-Myers Squibb, Celltrion, Epirus, Hospira, Merck-Serono, MSD, Orion Pharma, Pfizer, and UCB; no other relationships or activities that could appear to have influenced the submitted work.
Figures
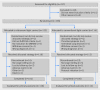
Fig 1 Protocol for escalation of disease modifying anti-rheumatic (DMARD) treatment. If patient responds or reaches target, current treatment is continued. *In patients with high disease activity and risk factors for progressive joint destruction, rescue option is available which includes moving to step 5 (introduce first biologic). †This step requires signs of ongoing inflammatory activity. TNF=tumour necrosis factor
Fig 2 Trial profile. DMARD=disease modifying anti-rheumatic drug; RA=rheumatoid arthritis
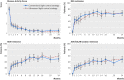
Fig 3 Top left: Disease Activity Score (DAS) over 24 months; least square mean estimates of DAS at all visits derived from mixed effects longitudinal model adjusted for baseline value and stratification factors. Top right: proportion of patients who achieved DAS remission over 24 months derived from logistic regression model. Bottom left: proportion of patients who achieved Simplified Disease Activity Index (SDAI) remission over 24 months derived from logistic regression model. Bottom right: proportion of patients who achieved American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) boolean remission over 24 months from logistic regression model. Bars indicate 95% confidence limits
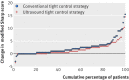
Fig 4 Cumulative probability plot of change between baseline and 24 months in van der Heijde modified Sharp score
Similar articles
- Effect of Magnetic Resonance Imaging vs Conventional Treat-to-Target Strategies on Disease Activity Remission and Radiographic Progression in Rheumatoid Arthritis: The IMAGINE-RA Randomized Clinical Trial.
Møller-Bisgaard S, Hørslev-Petersen K, Ejbjerg B, Hetland ML, Ørnbjerg LM, Glinatsi D, Møller J, Boesen M, Christensen R, Stengaard-Pedersen K, Madsen OR, Jensen B, Villadsen JA, Hauge EM, Bennett P, Hendricks O, Asmussen K, Kowalski M, Lindegaard H, Nielsen SM, Bliddal H, Krogh NS, Ellingsen T, Nielsen AH, Balding L, Jurik AG, Thomsen HS, Østergaard M. Møller-Bisgaard S, et al. JAMA. 2019 Feb 5;321(5):461-472. doi: 10.1001/jama.2018.21362. JAMA. 2019. PMID: 30721294 Free PMC article. Clinical Trial. - Conventional versus ultrasound treat to target: no difference in magnetic resonance imaging inflammation or joint damage over 2 years in early rheumatoid arthritis.
Sundin U, Aga AB, Skare Ø, Nordberg LB, Uhlig T, Hammer HB, van der Heijde D, Kvien TK, Lillegraven S, Haavardsholm EA; ARCTIC study group. Sundin U, et al. Rheumatology (Oxford). 2020 Sep 1;59(9):2550-2555. doi: 10.1093/rheumatology/kez674. Rheumatology (Oxford). 2020. PMID: 31999341 Clinical Trial. - Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial.
van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FH, Kievit W, van Vollenhoven RF, Bijlsma JW, van den Bemt BJ, den Broeder AA. van Herwaarden N, et al. BMJ. 2015 Apr 9;350:h1389. doi: 10.1136/bmj.h1389. BMJ. 2015. PMID: 25858265 Free PMC article. Clinical Trial. - Evidence-based recommendations for the diagnosis and management of rheumatoid arthritis for non-rheumatologists: Integrating systematic literature research and expert opinion of the Thai Rheumatism Association.
Katchamart W, Narongroeknawin P, Chevaisrakul P, Dechanuwong P, Mahakkanukrauh A, Kasitanon N, Pakchotanon R, Sumethkul K, Ueareewongsa P, Ukritchon S, Bhurihirun T, Duangkum K, Intapiboon P, Intongkam S, Jangsombatsiri W, Jatuworapruk K, Kositpesat N, Leungroongroj P, Lomarat W, Petcharat C, Sittivutworapant S, Suebmee P, Tantayakom P, Tipsing W, Asavatanabodee P, Chiowchanwisawakit P, Foocharoen C, Koolvisoot A, Louthrenoo W, Siripaitoon B, Totemchokchyakarn K, Kitumnuaypong T; Thai Rheumatism Association. Katchamart W, et al. Int J Rheum Dis. 2017 Sep;20(9):1142-1165. doi: 10.1111/1756-185X.12905. Epub 2016 Jul 25. Int J Rheum Dis. 2017. PMID: 27452207 Review. - The role of ultrasound and magnetic resonance imaging for treat to target in rheumatoid arthritis and psoriatic arthritis.
Mandl P, Aletaha D. Mandl P, et al. Rheumatology (Oxford). 2019 Dec 1;58(12):2091-2098. doi: 10.1093/rheumatology/kez397. Rheumatology (Oxford). 2019. PMID: 31518423 Review.
Cited by
- The Role of Musculoskeletal Ultrasound in the Rheumatoid Arthritis Continuum.
Di Matteo A, Mankia K, Azukizawa M, Wakefield RJ. Di Matteo A, et al. Curr Rheumatol Rep. 2020 Jun 19;22(8):41. doi: 10.1007/s11926-020-00911-w. Curr Rheumatol Rep. 2020. PMID: 32562012 Free PMC article. Review. - Remission, response, retention and persistence to treatment with disease-modifying agents in patients with rheumatoid arthritis: a study of harmonised Swedish, Danish and Norwegian cohorts.
Westerlind H, Glintborg B, Hammer HB, Saevarsdottir S, Krogh NS, Hetland ML, Hauge EM, Martinez Tejada I, Sexton J, Askling J. Westerlind H, et al. RMD Open. 2023 Sep;9(3):e003027. doi: 10.1136/rmdopen-2023-003027. RMD Open. 2023. PMID: 37673441 Free PMC article. - [S2e guideline: treatment of rheumatoid arthritis with disease-modifying drugs].
Fiehn C, Holle J, Iking-Konert C, Leipe J, Weseloh C, Frerix M, Alten R, Behrens F, Baerwald C, Braun J, Burkhardt H, Burmester G, Detert J, Gaubitz M, Gause A, Gromnica-Ihle E, Kellner H, Krause A, Kuipers J, Lorenz HM, Müller-Ladner U, Nothacker M, Nüsslein H, Rubbert-Roth A, Schneider M, Schulze-Koops H, Seitz S, Sitter H, Specker C, Tony HP, Wassenberg S, Wollenhaupt J, Krüger K. Fiehn C, et al. Z Rheumatol. 2018 Aug;77(Suppl 2):35-53. doi: 10.1007/s00393-018-0481-y. Z Rheumatol. 2018. PMID: 29968101 Review. German. - Fatigue in patients with early rheumatoid arthritis undergoing treat-to-target therapy: predictors and response to treatment.
Holten K, Paulshus Sundlisater N, Lillegraven S, Sexton J, Nordberg LB, Moholt E, Hammer HB, Uhlig T, Kvien TK, Haavardsholm EA, Aga AB. Holten K, et al. Ann Rheum Dis. 2022 Mar;81(3):344-350. doi: 10.1136/annrheumdis-2021-220750. Epub 2021 Aug 13. Ann Rheum Dis. 2022. PMID: 34389605 Free PMC article.
References
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263-9. 10.1016/S0140-6736(04)16676-2 pmid:15262104. - DOI - PubMed
- Verstappen SM, Jacobs JW, van der Veen MJ, et al. Utrecht Rheumatoid Arthritis Cohort study group. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 2007;66:1443-9. 10.1136/ard.2007.071092 pmid:17519278. - DOI - PMC - PubMed
- Smolen JS, Aletaha D, Bijlsma JW, et al. T2T Expert Committee. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631-7. 10.1136/ard.2009.123919 pmid:20215140. - DOI - PMC - PubMed
- Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet 2009;373:659-72. 10.1016/S0140-6736(09)60008-8 pmid:19157532. - DOI - PubMed
- Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26-37. 10.1002/art.21519 pmid:16385520. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous