Punica granatum L. Hydrogel for Wound Care Treatment: From Case Study to Phytomedicine Standardization - PubMed (original) (raw)
Case Reports
Punica granatum L. Hydrogel for Wound Care Treatment: From Case Study to Phytomedicine Standardization
Aline Fleck et al. Molecules. 2016.
Abstract
The pharmacological activities of many Punica granatum L. components suggest a wide range of clinical applications for the prevention and treatment of diseases where chronic inflammation is believed to play an essential etiologic role. The current work reports a case study analyzing the effect produced by a magistral formulation of ethanolic extracts of Punica granatum peels on a non-healing chronic ulcer. The complete closure of the chronic ulcer that was initially not responsive to standard medical care was observed. A 2% (w/w) P. granatum peels ethanolic extract hydrogel-based formulation (PGHF) was standardized and subjected to physicochemical studies to establish the quality control parameters using, among others, assessment criteria such as optimum appearance, pH range, viscosity and hydrogel disintegration. The stability and quantitative chromatographic data was assessed in storage for six months under two temperature regimes. An efficient HPLC-DAD method was established distinguishing the biomarkers punicalin and punicalagin simultaneously in a single 8 min run. PGHF presented suitable sensorial and physicochemical performance, showing that punicalagin was not significantly affected by storage (p > 0.05). Formulations containing extracts with not less than 0.49% (w/w) total punicalagin might find good use in wound healing therapy.
Keywords: Punica granatum; hydrogel; punicalagin; quality control; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
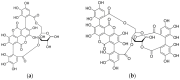
Chemical structures of the ellagitannins punicalin (a) and punicalagin (b).
Figure 2
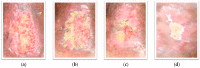
Photographs of the patient’s wound. PGMF: Punica granatum magistral formulation; (a): 1 day before PGMF treatment; (b): week 1 of PGMF treatment; (c): week 6 of PGMF treatment; (d): week 10 of PGMF treatment.
Figure 3
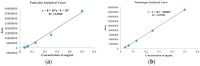
Analytical curves of the biomarkers (a) punicalin and (b) punicalagin.
Figure 4
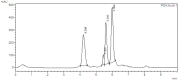
HPLC chromatographs of punicalin (a) and punicalagin (b) standards. Chromatograph was run on a Shimadzu LC-20AT HPLC equipped with a SPD-M20A PDA detection system. Separations were carried out on a Dr. Maisch GmbH C18 (Ammerbuch-Entringen, Germany) column (5 μm, 250 mm × 4.6 mm), 25 °C, with a mobile phase of 0.1% (v/v) aqueous solution of phosphoric acid and acetonitrile (80:20). The mobile phase flow rate was 1 mL/min with 10 min run time. The standards were detected using a wavelength of 254 nm. Sample injection volume was 10 μL. Retention times for punicalin and punicalagin were 4.203 and 5.987 min, respectively.
Figure 5
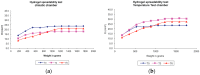
Spreadability test of hydrogel-based formulation of ethanolic extract of P. granatum peels (2% w/w) in different storage conditions. (a) Climatic chamber; (b) Temperature test chamber; T0: after production; T3: 3 months; T6: 6 months. Results are represented by means (n = 3).
Figure 6
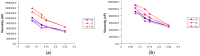
Share stress—share rate curves of hydrogel-based formulation of ethanolic extract of P. granatum (2%, w/w); T0: after production, T3: 3 months and T6: 6 months of storage under different storage conditions. (a) Temperature test chamber; (b) Climatic chamber. Results are represented by means (n = 3).
Figure 7
Viscosity—share rate curves of hydrogel-based formulation of ethanolic extract of P. granatum (2%, w/w); T0: after production, T3: 3 months and T6: 6 months of storage at different storage conditions. (a) Climatic chamber; (b) Temperature test chamber. Results are represented by means (n = 3).
Figure 8
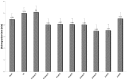
Disintegration time of hydrogel-base formulations. Blank; after production (T0); temperature test chamber-polyethylene-time: 3 months (TTCPolyT3); temperature test chamber-polyethylene-time: 6 months (TTCPolyT6); temperature test chamber-aluminum-time: 3 months (TTCAlT3); temperature test chamber-aluminum-time: 6 months (TTCAlT6); climatic chamber-polyethylene-time: 3 months (CCPolyT3); climatic chamber-polyethylene-time 3months (CCPolyT6); climatic chamber-aluminum-time: 3 months (CCAlT3); climatic chamber-aluminum-time: 6 months (CCAlT6). Results are represented by means ± S.E.M. (n = 3).
Figure 9
HPLC chromatographs of Punica granatum peel ethanolic extract hydrogel-based formulation (PGHF), showing retention times for punicalin (4.203 min) and punicalagin (5.987 min). Chromatography was run on a Shimadzu LC-20AT HPLC with SPD-M20A PDA detection. Separations were carried out on a Dr. Maisch GmbH C18 column (5 μm, 250 mm × 4.6 mm), 25 °C, with a mobile phase of 0.1% (v/v) aqueous solution of phosphoric acid and acetonitrile (80:20). The mobile phase flow rate was 1 mL/min with 10 min run time. The standards were detected using a wavelength of 254 nm. Sample injection volume was 10 μL.
Figure 10
Punicalagin (a) and punicalin (b) contents in PGHF; HGT3TTC-hydrogel, time: 3 months, temperature test chamber; HGT6TTC-hydrogel, time: 6 months, temperature test chamber; HGT3CC -hydrogel, time: 3 months, climate chamber; HGT6CC-hydrogel, time: 6 months, climate chamber. Results are represented by means ± S.E.M. (n = 3). No statistical significant difference was detected. Statistical analysis was performed by two-way ANOVA followed by Bonferroni’s test of multiple comparisons (p < 0.05).
Similar articles
- The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica Granatum L. (Pomegranate) as a Functional Food in Humans and Animals.
Saeed M, Naveed M, BiBi J, Kamboh AA, Arain MA, Shah QA, Alagawany M, El-Hack MEA, Abdel-Latif MA, Yatoo MI, Tiwari R, Chakraborty S, Dhama K. Saeed M, et al. Recent Pat Inflamm Allergy Drug Discov. 2018;12(1):24-38. doi: 10.2174/1872213X12666180221154713. Recent Pat Inflamm Allergy Drug Discov. 2018. PMID: 29473532 Review. - Study on wound healing activity of Punica granatum peel.
Murthy KN, Reddy VK, Veigas JM, Murthy UD. Murthy KN, et al. J Med Food. 2004 Summer;7(2):256-9. doi: 10.1089/1096620041224111. J Med Food. 2004. PMID: 15298776 - Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds.
Hayouni EA, Miled K, Boubaker S, Bellasfar Z, Abedrabba M, Iwaski H, Oku H, Matsui T, Limam F, Hamdi M. Hayouni EA, et al. Phytomedicine. 2011 Aug 15;18(11):976-84. doi: 10.1016/j.phymed.2011.02.011. Epub 2011 Apr 3. Phytomedicine. 2011. PMID: 21466954 - Preparation and characterisation of Punica granatum pericarp aqueous extract loaded chitosan-collagen-starch membrane: role in wound healing process.
Amal B, Veena B, Jayachandran VP, Shilpa J. Amal B, et al. J Mater Sci Mater Med. 2015 May;26(5):181. doi: 10.1007/s10856-015-5515-2. Epub 2015 Apr 17. J Mater Sci Mater Med. 2015. PMID: 25893391 - Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer.
Lansky EP, Newman RA. Lansky EP, et al. J Ethnopharmacol. 2007 Jan 19;109(2):177-206. doi: 10.1016/j.jep.2006.09.006. Epub 2006 Sep 10. J Ethnopharmacol. 2007. PMID: 17157465 Review.
Cited by
- Effects of Methanolic Extract Based-Gel From Saudi Pomegranate Peels With Enhanced Healing Potential on Excision Wounds in Diabetic Rats.
Karim S, Alkreathy HM, Ahmad A, Khan MI. Karim S, et al. Front Pharmacol. 2021 May 28;12:704503. doi: 10.3389/fphar.2021.704503. eCollection 2021. Front Pharmacol. 2021. PMID: 34122120 Free PMC article. - Antibacterial smart hydrogels: New hope for infectious wound management.
Aliakbar Ahovan Z, Esmaeili Z, Eftekhari BS, Khosravimelal S, Alehosseini M, Orive G, Dolatshahi-Pirouz A, Pal Singh Chauhan N, Janmey PA, Hashemi A, Kundu SC, Gholipourmalekabadi M. Aliakbar Ahovan Z, et al. Mater Today Bio. 2022 Nov 19;17:100499. doi: 10.1016/j.mtbio.2022.100499. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36466959 Free PMC article. Review. - Evaluation of the In Vitro Oral Wound Healing Effects of Pomegranate (Punica granatum) Rind Extract and Punicalagin, in Combination with Zn (II).
Celiksoy V, Moses RL, Sloan AJ, Moseley R, Heard CM. Celiksoy V, et al. Biomolecules. 2020 Aug 25;10(9):1234. doi: 10.3390/biom10091234. Biomolecules. 2020. PMID: 32854243 Free PMC article. - A Review of the Potential Benefits of Plants Producing Berries in Skin Disorders.
Piazza S, Fumagalli M, Khalilpour S, Martinelli G, Magnavacca A, Dell'Agli M, Sangiovanni E. Piazza S, et al. Antioxidants (Basel). 2020 Jun 20;9(6):542. doi: 10.3390/antiox9060542. Antioxidants (Basel). 2020. PMID: 32575730 Free PMC article. Review. - Assessment of the Anti-Inflammatory, Antibacterial and Anti-Aging Properties and Possible Use on the Skin of Hydrogels Containing Epilobium angustifolium L. Extracts.
Nowak A, Zagórska-Dziok M, Perużyńska M, Cybulska K, Kucharska E, Ossowicz-Rupniewska P, Piotrowska K, Duchnik W, Kucharski Ł, Sulikowski T, Droździk M, Klimowicz A. Nowak A, et al. Front Pharmacol. 2022 Jul 1;13:896706. doi: 10.3389/fphar.2022.896706. eCollection 2022. Front Pharmacol. 2022. PMID: 35846995 Free PMC article.
References
- Saraf S. Formulating moisturizers using natural raw materials. In: Lodén M., Maibach H.I., editors. Treatment of Dry Skin Syndrome. 1st ed. Springer-Verlag; Berlin, Germany: 2012. pp. 379–397.
- Kaneria M.J., Bapodara M.B., Chanda S.V. Effect of extraction techniques and solvents on antioxidant activity of pomegranate (Punica granatum L.) leaf and stem. Food Anal. Method. 2012;5:396–404. doi: 10.1007/s12161-011-9257-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical