An Update on the Appropriate Role for Hyperbaric Oxygen: Indications and Evidence - PubMed (original) (raw)
Review
An Update on the Appropriate Role for Hyperbaric Oxygen: Indications and Evidence
Caroline E Fife et al. Plast Reconstr Surg. 2016 Sep.
Abstract
Background: Among advanced therapeutic interventions for wounds, hyperbaric oxygen therapy (HBOT) has the unique ability to ameliorate tissue hypoxia, reduce pathologic inflammation, and mitigate ischemia reperfusion injury. Most of the conditions for which it is utilized have few successful alternative treatments, and the morbidity and mortality associated with treatment failure are significant. Data on the efficacy and effectiveness of HBOT were reviewed, comparative effectiveness research of HBOT was explained, and a new paradigm for the appropriate use of HBOT was described.
Methods: Systematic reviews and randomized controlled trials that have evaluated HBOT were reviewed.
Results: Although numerous small randomized controlled trials provide compelling support for HBOT, the physics of the hyperbaric environment create significant barriers to trial design. The electronic health record infrastructure created to satisfy mandatory quality and registry reporting requirements as part of healthcare reform can be harnessed to facilitate the acquisition of real world data for HBOT comparative effectiveness studies and clinical decision support.
Conclusions: Predictive models can identify patients unlikely to heal spontaneously and most likely to benefit from HBOT. Although electronic health records can automate the calculation of predictive models making them available at the point of care, using them in clinical decision making is complicated. It is not clear whether stakeholders will support the allocation of healthcare resources using mathematical models, but the current patient selection process mandates a 30-day delay for all patients who might benefit and allows treatment for at least some patients who cannot benefit.
Conflict of interest statement
Dr. Fife is the Executive Director of the Chronic Disease Registry (CDR), a 501(c)(3) nonprofit organization. The registry operates the U.S. Wound Registry, which is recognized by the Centers for Medicare and Medicaid Services as a qualified clinical data registry. Dr. Fife received some grant support from KCI, an ACELITY company. Ms. Eckert and Carter are paid consultants of Dr. Fife for this study and assisted in the preparation of the article.
Figures
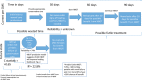
Fig. 1.
Choosing Wisely for HBOT in DFUs: a mathematical paradigm.,,, TcP
o
2 = transcutaneous oximetry measurement; R2 is the coefficient of determination, a statistical measure of how close data are to the fitted regression line. R2 is always between 0% and 100%, whereby 0% indicates that the model explains none of the variability of the data around its mean, and 100% indicates that the model explains all such variability. A very low R2 is not desirable in models that might be used to influence clinical decisions regarding the use of a medical intervention. However, even in this model with a relatively low R2 but statistically significant predictors, it is still possible to understand how changes in the predictor values are associated with the changes in the likelihood of benefit from HBOT; C-statistic is the probability that predicting the outcome is better than chance. C-statistic is used to compare the goodness of fit of logistic regression models. Values for this measure range from 0.5 to 1.0, whereby a value of 0.5 indicates that the model is no better than chance at making a prediction, and a value of 1.0 indicates that the model is perfect at identifying the desired outcome. Models are typically considered reasonable when the C-statistic is higher than 0.7 and strong when C exceeds 0.8. Therefore, a C-statistic >0.65 for this model predicting the benefit of HBOT is better than chance, but is not a strong model.
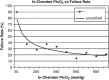
Fig. 2.
In-chamber failure rates vs Ptc
o
2 achievement. Data from 221 patients with DFUs. This figure demonstrates that for Ptc
o
2 levels ≤100, the failure rate was 90.0%; for Ptc
o
2 levels of 101–200 and for 301–400, the failure rate was 35.7%; for Ptc
o
2 levels >1000, the failure rate was 18.2%.
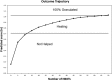
Fig. 3.
This figure depicts the expected outcome trajectory of a hypothetical patient with the following patient characteristics: age = 60 yr; diabetes = 15 yr; Wagner Grade = 3; Ptc
o
2 (air) = 15 mm Hg; nonsmoker. Some improvement in the wound should be visible after 12 hyperbaric treatments, but approximately 35–40 treatments will likely be required to achieve healing. The curve demonstrates that there is little incremental benefit from additional treatments after 40. The clinical use of predictive models like this would require significant provider education to ensure that they are not interpreted as literal recommendations. For example, if the model predicted that 200 treatments would be necessary to achieve improvement, the clinician should understand that benefit from HBOT is not likely to be achieved within a clinically reasonable course of treatment. Conversely, if the model were to predict, for example, that a patient will require fewer than 10 treatments to heal, it is likely that the patient does not need any hyperbaric treatments at all.
Comment in
- Discussion: An Update on the Appropriate Role for Hyperbaric Oxygen: Indications and Evidence.
Guilliod RR, Pompeo MQ. Guilliod RR, et al. Plast Reconstr Surg. 2016 Sep;138(3 Suppl):117S-118S. doi: 10.1097/PRS.0000000000002700. Plast Reconstr Surg. 2016. PMID: 27556751 No abstract available.
Similar articles
- Poorly designed research does not help clarify the role of hyperbaric oxygen in the treatment of chronic diabetic foot ulcers.
Mutluoglu M, Uzun G, Bennett M, Germonpré P, Smart D, Mathieu D. Mutluoglu M, et al. Diving Hyperb Med. 2016 Sep;46(3):133-134. Diving Hyperb Med. 2016. PMID: 27723012 - Hyperbaric oxygen therapy: solution for difficult to heal acute wounds? Systematic review.
Eskes AM, Ubbink DT, Lubbers MJ, Lucas C, Vermeulen H. Eskes AM, et al. World J Surg. 2011 Mar;35(3):535-42. doi: 10.1007/s00268-010-0923-4. World J Surg. 2011. PMID: 21184071 Free PMC article. Review. - Hyperbaric oxygen therapy for chronic wounds.
Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Kranke P, et al. Cochrane Database Syst Rev. 2004;(2):CD004123. doi: 10.1002/14651858.CD004123.pub2. Cochrane Database Syst Rev. 2004. PMID: 15106239 Updated. Review. - Hyperbaric oxygen for chronic wounds.
Goldstein LJ. Goldstein LJ. Dermatol Ther. 2013 May-Jun;26(3):207-14. doi: 10.1111/dth.12053. Dermatol Ther. 2013. PMID: 23742281 Review. - A three species model to simulate application of Hyperbaric Oxygen Therapy to chronic wounds.
Flegg JA, McElwain DL, Byrne HM, Turner IW. Flegg JA, et al. PLoS Comput Biol. 2009 Jul;5(7):e1000451. doi: 10.1371/journal.pcbi.1000451. Epub 2009 Jul 31. PLoS Comput Biol. 2009. PMID: 19649306 Free PMC article.
Cited by
- Combination of Hyperbaric Oxygen Therapy and Oral Steroids for the Treatment of Sudden Sensorineural Hearing Loss: Early or Late?
Cavaliere M, De Luca P, Scarpa A, Strzalkowski AM, Ralli M, Calvanese M, Savignano L, Viola P, Cassandro C, Chiarella G, Di Stadio A. Cavaliere M, et al. Medicina (Kaunas). 2022 Oct 10;58(10):1421. doi: 10.3390/medicina58101421. Medicina (Kaunas). 2022. PMID: 36295581 Free PMC article. Clinical Trial. - Randomized controlled clinical trial evaluating the efficacy of hyperbaric oxygen therapy in facilitating the healing of chronic foot ulcers in diabetic patients: the study protocol.
Lopes JRA, D'Agostino Dias M, Correa JA, Batalha MAB, Guerra LKD. Lopes JRA, et al. Trials. 2020 Sep 29;21(1):816. doi: 10.1186/s13063-020-04757-6. Trials. 2020. PMID: 32993766 Free PMC article. - Global outcomes and lessons learned in the management of Fournier's gangrene from high-volume centres: findings from a literature review over the last two decades.
Bowen D, Juliebø-Jones P, Somani BK. Bowen D, et al. World J Urol. 2022 Oct;40(10):2399-2410. doi: 10.1007/s00345-022-04139-4. Epub 2022 Sep 4. World J Urol. 2022. PMID: 36059020 Review. - Hyperbaric oxygen in animal model of rheumatoid arthritis: Analysis Of HIF-1α, ACPA and IL-17a.
Harnanik T, Prihartono S, Juliandhy T. Harnanik T, et al. Infect Dis Rep. 2020 Jul 6;12(Suppl 1):8766. doi: 10.4081/idr.2020.8766. eCollection 2020 Jul 7. Infect Dis Rep. 2020. PMID: 32874480 Free PMC article. - Quality of evidence supporting the role of hyperbaric oxygen therapy for diabetic foot ulcers.
Jiang F, Zhang Y, Cheng S, Yang X, Bai M, Zhang M. Jiang F, et al. Int Wound J. 2024 Apr;21(4):e14530. doi: 10.1111/iwj.14530. Epub 2023 Dec 6. Int Wound J. 2024. PMID: 38053520 Free PMC article. Review.
References
- Fife CE, Hopf H. Discussion. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(Suppl 1):142S–143S. - PubMed
- Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. - PubMed
- Doctor N, Pandya S, Supe A. Hyperbaric oxygen therapy in diabetic foot. J Postgrad Med. 1992;38:112–4, 111. - PubMed
- Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19:1338–1343. - PubMed
- Lin T, Chen S, Niu K. The vascular effects of hyperbaric oxygen therapy in treatment of early diabetic foot. Undersea Hyperb Med. 2001;28(Suppl):67.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical