Replication of human noroviruses in stem cell-derived human enteroids - PubMed (original) (raw)
. 2016 Sep 23;353(6306):1387-1393.
doi: 10.1126/science.aaf5211. Epub 2016 Aug 25.
Sue E Crawford 1, Kosuke Murakami 1, James R Broughman 1, Umesh Karandikar 1, Victoria R Tenge 1, Frederick H Neill 1, Sarah E Blutt 1, Xi-Lei Zeng 1, Lin Qu 1, Baijun Kou 1, Antone R Opekun 2, Douglas Burrin 3, David Y Graham 4, Sasirekha Ramani 1, Robert L Atmar 5, Mary K Estes 6
Affiliations
- PMID: 27562956
- PMCID: PMC5305121
- DOI: 10.1126/science.aaf5211
Replication of human noroviruses in stem cell-derived human enteroids
Khalil Ettayebi et al. Science. 2016.
Abstract
The major barrier to research and development of effective interventions for human noroviruses (HuNoVs) has been the lack of a robust and reproducible in vitro cultivation system. HuNoVs are the leading cause of gastroenteritis worldwide. We report the successful cultivation of multiple HuNoV strains in enterocytes in stem cell-derived, nontransformed human intestinal enteroid monolayer cultures. Bile, a critical factor of the intestinal milieu, is required for strain-dependent HuNoV replication. Lack of appropriate histoblood group antigen expression in intestinal cells restricts virus replication, and infectivity is abrogated by inactivation (e.g., irradiation, heating) and serum neutralization. This culture system recapitulates the human intestinal epithelium, permits human host-pathogen studies of previously noncultivatable pathogens, and allows the assessment of methods to prevent and treat HuNoV infections.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
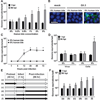
Fig. 1
Replication of GII.4 variants in human intestinal enteroids. Jejunal HIE monolayers were inoculated with (A) 9×105 genome equivalents of the indicated HuNoV GII.4 stool filtrates. RNA was extracted from cells and medium, and viral genome equivalents quantified by RT-qPCR. RNA at 1 hpi was collected after removal of virus inoculum and washing of cells twice to remove any unattached viruses. Each data bar represents the mean of three wells of inoculated HIEs. Error bars denote standard deviation. Each experiment was performed two or more times, with three technical replicates in each experiment. Panels (B-E) and (F) represent monolayers inoculated with 9×107 and 9×105 genome equivalents of GII.4/2012-1, respectively. (B) Expression of VP1 was detected in enterocytes (villin, red) in formalin-fixed, paraffin-embedded enteroid monolayer sections using antibody against GII.4/2012 VLPs (green). DAPI detects nuclei (blue). Scale bar = 25 µm. (C) Flow cytometry quantitation and immunofluorescent detection of infected cells. Scale bar = 100 µm. (D) Electron micrograph of HuNoV particles from the supernatant of infected HIEs. Scale bar = 50 nm. Inset: small particle. Scale bar = 25 nm. (E) Western blot detecting polyprotein processing and VP1 expression. Asterisk marks a non-specific band. (F) Kinetics of HuNoV yield at the indicated time points. (G) Passaging of GII.4/2009 HuNoV in jejunal HIEs. (F, G) Viral genome equivalents quantified by RT-qPCR as indicated for panel A.
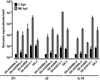
Fig. 2
Bile is required for GII.3 HuNoV replication and affects the cells. Jejunal HIE monolayers were pretreated (A-C) with the indicated concentrations of human bile for 2 days and then inoculated with GII.3 stool filtrate [(A, C) 4.3×105 or (B) 4.3×107 genome equivalents] and incubated with the same bile concentrations as used for pretreatment (see supplementary methods). (B) VP1 was detected in methanol-fixed monolayers at 24 hpi using guinea pig anti-GII.3 VLP antiserum (green) and DAPI to detect nuclei (blue). Scale bar = 25 µm. (D) To determine if the effect of bile was on the virus or the cells, the virus was either not treated or treated with 5% human bile for 1 hour at 37°C, and then diluted to decrease the bile concentration to 0.025% prior to infection of HIE monolayers not pretreated with bile. Alternatively, cells were either not treated or treated with 5% human bile for 2 days prior to and during infection. Inoculations were performed with 4.3×105 genome equivalents. (E) Schematic showing with black arrows when bile was added to HIEs for the experiment shown in (F). For A, C, D and F, genome equivalents were determined as indicated in Fig. 1. Error bars denote standard deviation. *, P< 0.05 comparing genome equivalents to 1 hpi.
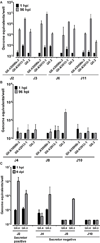
Fig. 3
GII.4 variants and GII.3 HuNoVs replicate in HIEs generated from different intestinal segments. Duodenal (D1), jejunal (J2), and ileal (IL16) HIEs were treated with 1% sow bile for the GII.4 variants or 5% human bile for GII.3 for 48 hours, and then inoculated with the indicated HuNoVs (GII.4/2006b-2, GII.4/2009, GII.4/2012-1: 9×105; GII.4/2006b-3: 5.5×105; GII.3: 4.3×105 genome equivalents) and cultured in the presence of bile. Genome equivalents were determined as indicated in Fig. 1. Error bars denote standard deviation.
Fig. 4
Replication of GII.4 strains but not GII.3 depends on HIE secretor status. (A) Secretor positive jejunal (J2, J3, J6 and J11) or (B) secretor negative jejunal (J4, J8 and J10) HIEs were inoculated with the indicated GII.4 or GII.3 HuNoVs (with the same amounts of genome equivalents as indicated in Fig. 3) in the presence of bile (1% sow bile for GII.4 variants or 5% human bile for GII.3) for 96 hours. At 96 hpi, GII.4 strains replicate in secretor positive HIEs but not secretor negative lines, while GII.3 replicates in all secretor positive HIEs and one secretor negative line (J8). (C) At 6 dpi, the GII.3 virus shows replication in an additional secretor negative HIE (J4) while no growth of GII.4/2012-1 virus is seen. A secretor positive J2 HIE is included as control to show replication of GII.4 at 6 dpi. (A–C) Genome equivalents were determined as indicated in Fig. 1. Error bars denote standard deviation.
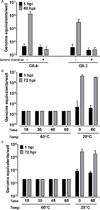
Fig. 5
Inactivation of GII.4 and GII.3 HuNoV infectivity by gamma irradiation and heat treatment. (A) GII.4/2012-1 and GII.3 HuNoVs were gamma irradiated or incubated at room temperature overnight. (B) GII.4/2012-1 or (C) GII.3 (9×105 and 4.3×105 genome equivalents, respectively) were heat-inactivated at 60°C for the indicated time points or incubated at room temperature for 0 and 60 minutes. Jejunal HIEs were inoculated with each sample. Genome equivalents were determined as indicated in Fig. 1. Error bars denote standard deviation.
Comment in
- COVID-19: organoids go viral.
Clevers H. Clevers H. Nat Rev Mol Cell Biol. 2020 Jul;21(7):355-356. doi: 10.1038/s41580-020-0258-4. Nat Rev Mol Cell Biol. 2020. PMID: 32483314 Free PMC article.
Similar articles
- Human Norovirus Cultivation in Nontransformed Stem Cell-Derived Human Intestinal Enteroid Cultures: Success and Challenges.
Estes MK, Ettayebi K, Tenge VR, Murakami K, Karandikar U, Lin SC, Ayyar BV, Cortes-Penfield NW, Haga K, Neill FH, Opekun AR, Broughman JR, Zeng XL, Blutt SE, Crawford SE, Ramani S, Graham DY, Atmar RL. Estes MK, et al. Viruses. 2019 Jul 11;11(7):638. doi: 10.3390/v11070638. Viruses. 2019. PMID: 31336765 Free PMC article. Review. - Insights into human norovirus cultivation in human intestinal enteroids.
Ettayebi K, Kaur G, Patil K, Dave J, Ayyar BV, Tenge VR, Neill FH, Zeng X-L, Speer AL, Di Rienzi SC, Britton RA, Blutt SE, Crawford SE, Ramani S, Atmar RL, Estes MK. Ettayebi K, et al. mSphere. 2024 Nov 21;9(11):e0044824. doi: 10.1128/msphere.00448-24. Epub 2024 Oct 15. mSphere. 2024. PMID: 39404443 Free PMC article. - New Insights and Enhanced Human Norovirus Cultivation in Human Intestinal Enteroids.
Ettayebi K, Tenge VR, Cortes-Penfield NW, Crawford SE, Neill FH, Zeng XL, Yu X, Ayyar BV, Burrin D, Ramani S, Atmar RL, Estes MK. Ettayebi K, et al. mSphere. 2021 Jan 27;6(1):e01136-20. doi: 10.1128/mSphere.01136-20. mSphere. 2021. PMID: 33504663 Free PMC article. - Antiviral Activity of Olanexidine-Containing Hand Rub against Human Noroviruses.
Ettayebi K, Salmen W, Imai K, Hagi A, Neill FH, Atmar RL, Prasad BVV, Estes MK. Ettayebi K, et al. mBio. 2022 Apr 26;13(2):e0284821. doi: 10.1128/mbio.02848-21. Epub 2022 Mar 17. mBio. 2022. PMID: 35297675 Free PMC article. - Human norovirus cultivation systems and their use in antiviral research.
Hayashi T, Kobayashi S, Hirano J, Murakami K. Hayashi T, et al. J Virol. 2024 Apr 16;98(4):e0166323. doi: 10.1128/jvi.01663-23. Epub 2024 Mar 12. J Virol. 2024. PMID: 38470106 Free PMC article. Review.
Cited by
- Antigenic cartography reveals complexities of genetic determinants that lead to antigenic differences among pandemic GII.4 noroviruses.
Kendra JA, Tohma K, Ford-Siltz LA, Lepore CJ, Parra GI. Kendra JA, et al. Proc Natl Acad Sci U S A. 2021 Mar 16;118(11):e2015874118. doi: 10.1073/pnas.2015874118. Proc Natl Acad Sci U S A. 2021. PMID: 33836574 Free PMC article. - Microbiota Modulation of the Gut-Lung Axis in COVID-19.
de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRB. de Oliveira GLV, et al. Front Immunol. 2021 Feb 24;12:635471. doi: 10.3389/fimmu.2021.635471. eCollection 2021. Front Immunol. 2021. PMID: 33717181 Free PMC article. Review. - Enteroaggregative E. coli Adherence to Human Heparan Sulfate Proteoglycans Drives Segment and Host Specific Responses to Infection.
Rajan A, Robertson MJ, Carter HE, Poole NM, Clark JR, Green SI, Criss ZK, Zhao B, Karandikar U, Xing Y, Margalef-Català M, Jain N, Wilson RL, Bai F, Hyser JM, Petrosino J, Shroyer NF, Blutt SE, Coarfa C, Song X, Prasad BV, Amieva MR, Grande-Allen J, Estes MK, Okhuysen PC, Maresso AW. Rajan A, et al. PLoS Pathog. 2020 Sep 28;16(9):e1008851. doi: 10.1371/journal.ppat.1008851. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32986782 Free PMC article. - Three-Dimensional Regeneration of Patient-Derived Intestinal Organoid Epithelium in a Physiodynamic Mucosal Interface-on-a-Chip.
Shin YC, Shin W, Koh D, Wu A, Ambrosini YM, Min S, Eckhardt SG, Fleming RYD, Kim S, Park S, Koh H, Yoo TK, Kim HJ. Shin YC, et al. Micromachines (Basel). 2020 Jul 7;11(7):663. doi: 10.3390/mi11070663. Micromachines (Basel). 2020. PMID: 32645991 Free PMC article. - Tobamoviruses can be frequently present in the oropharynx and gut of infants during their first year of life.
Aguado-García Y, Taboada B, Morán P, Rivera-Gutiérrez X, Serrano-Vázquez A, Iša P, Rojas-Velázquez L, Pérez-Juárez H, López S, Torres J, Ximénez C, Arias CF. Aguado-García Y, et al. Sci Rep. 2020 Aug 12;10(1):13595. doi: 10.1038/s41598-020-70684-w. Sci Rep. 2020. PMID: 32788688 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- U19 AI116497/AI/NIAID NIH HHS/United States
- P30 CA125123/CA/NCI NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- P30 AI036211/AI/NIAID NIH HHS/United States
- P01 AI057788/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical