Technologies for imaging neural activity in large volumes - PubMed (original) (raw)
Review
Technologies for imaging neural activity in large volumes
Na Ji et al. Nat Neurosci. 2016.
Abstract
Neural circuitry has evolved to form distributed networks that act dynamically across large volumes. Conventional microscopy collects data from individual planes and cannot sample circuitry across large volumes at the temporal resolution relevant to neural circuit function and behaviors. Here we review emerging technologies for rapid volume imaging of neural circuitry. We focus on two critical challenges: the inertia of optical systems, which limits image speed, and aberrations, which restrict the image volume. Optical sampling time must be long enough to ensure high-fidelity measurements, but optimized sampling strategies and point-spread function engineering can facilitate rapid volume imaging of neural activity within this constraint. We also discuss new computational strategies for processing and analyzing volume imaging data of increasing size and complexity. Together, optical and computational advances are providing a broader view of neural circuit dynamics and helping elucidate how brain regions work in concert to support behavior.
Figures
Fig. 1
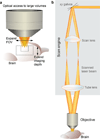
Optical access and basic optical layout of a 2PLSM. (a) Obtaining optical access to large brain volumes entails expanding the field of view (FOV) and extending the imaging depth. (b) A 2PLSM is comprised of a scan engine and an objective. In the scan engine, rapidly movable mirrors (galvanometer beam scanners, xy Galvos) reflect the excitation laser beam across a range of angles that are relayed using a scan lens and tube lens to the objective.
Fig. 2
Scan engine and objective determine imaging FOV. (a) Scan engines expand the excitation beam and rapidly vary the incidence angle on the back aperture of the objective (Obj) to create a scan pattern. A conventional approach involves beam scanners followed by a scan lens (SL) and a tube lens (TL). (b) The SL is placed at a distance equal to its focal length (FL) from the beam scanner (SLFL). The TL is placed at a distance equal to its FL from the Obj (TLFL). The beam diameter is expanded by a factor of TLFL/SLFL and the beam scanner scan angle (Ω1) is reduced by the reciprocal factor (SLFL/TLFL) to a smaller angle (Ω2). To use the full resolution of the Obj, the beam must be expanded to overfill the back aperture, and this expansion reduces the scan angle at the objective and can reduce the FOV. (c) Half of the width of the FOV (FOV1/2) is equal to Obj focal length (ObjFL, which is commonly expressed as a magnification factor, rather than a FL, by commercial vendors) multiplied by tan(Ω2). (d) FOV increases more rapidly with Obj back aperture diameter for lower NA optics. This relationship is illustrated using the parfocal approximation, and performance of real world systems can vary from the traces illustrated here, but the general relationship still applies.
Fig. 3
2D and 3D scanning strategies. (a) Focus is scanned in the xy plane by varying the direction of the excitation laser at the back pupil of the objective. (b) Focus is moved along z axis by moving the objective relative to the sample. (c) With a stationary imaging objective, focal shift in z can be achieved by changing the divergence of the laser beam. (d) With two objectives and a movable mirror, z position of the focus can be varied without incurring optical aberrations. (e) Multiple foci can be generated by manipulating the laser wavefront, which allows speed increase via multiplexing. (f) Volume can be imaged with an elongated focus with extended depth of field.
Fig. 4
Sampling strategies. (a) Raster scanning evenly samples a single plane of a volume. The scanning can be unidirectional or bidirectional, the latter offers higher speed, but may require additional image processing to reduce artifacts. Volumes can be imaged using multiple raster scanned planes. (b) Arbitrary line scanning can more optimally sample a volume. A geometric approach can be used with no prior knowledge of the anatomy, to sparsely sample a volume. Targeted scanning can use a previously acquired, raster-scanned, multiplane volume, as in a to target an arbitrary line scan to sample specific neurons. (c) Temporal multiplexing involves multiple beams scanning the volume with slight temporal delays between their laser pulses. Fluorescence events are attributed to specific beam if they occur in a small time window after excitation by that beam’s laser pulse, to ensure minimal crosstalk between multiplexed beams. Multi-foci scanning involves splitting laser power of each pulse between multiple foci. There is complete crosstalk between signals from the two pathways, but given sufficient sparsity, it is possible to demix signals from different neurons with high fidelity.
Fig. 5
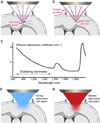
Aberration and scattering limit imaging depth. (a) Brain distorts the wavefront of the excitation light and leads to an aberrated focus (formed by orange rays), lowering image resolution and brightness. (b) Shaping the wavefront with adaptive optics cancels out brain-induced aberrations and recovers an ideal, diffraction-limited focus (formed by red rays). Green rays illustrate ideal imaging condition where the brain does not change ray directions. (c) The wavelength dependence of the effective attenuation coefficient (1/mm) (modified from reference 129) indicates that optimal excitation wavelength windows are near 1,300 and 1,700 nm. (d) and (e) Longer wavelength excitation light penetrates scattering brains more effectively than shorter wavelength ones.
Similar articles
- Diesel2p mesoscope with dual independent scan engines for flexible capture of dynamics in distributed neural circuitry.
Yu CH, Stirman JN, Yu Y, Hira R, Smith SL. Yu CH, et al. Nat Commun. 2021 Nov 17;12(1):6639. doi: 10.1038/s41467-021-26736-4. Nat Commun. 2021. PMID: 34789723 Free PMC article. - Random-Access Multiphoton Microscopy for Fast Three-Dimensional Imaging.
Reddy GD, Cotton RJ, Tolias AS, Saggau P. Reddy GD, et al. Adv Exp Med Biol. 2015;859:455-72. doi: 10.1007/978-3-319-17641-3_18. Adv Exp Med Biol. 2015. PMID: 26238064 Review. - Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy.
Keller PJ, Ahrens MB. Keller PJ, et al. Neuron. 2015 Feb 4;85(3):462-83. doi: 10.1016/j.neuron.2014.12.039. Neuron. 2015. PMID: 25654253 Review. - Two-photon imaging of neural population activity in zebrafish.
Renninger SL, Orger MB. Renninger SL, et al. Methods. 2013 Aug 15;62(3):255-67. doi: 10.1016/j.ymeth.2013.05.016. Epub 2013 May 31. Methods. 2013. PMID: 23727462 Review. - A Guide to Emerging Technologies for Large-Scale and Whole-Brain Optical Imaging of Neuronal Activity.
Weisenburger S, Vaziri A. Weisenburger S, et al. Annu Rev Neurosci. 2018 Jul 8;41:431-452. doi: 10.1146/annurev-neuro-072116-031458. Epub 2018 Apr 25. Annu Rev Neurosci. 2018. PMID: 29709208 Free PMC article. Review.
Cited by
- Imaging volumetric dynamics at high speed in mouse and zebrafish brain with confocal light field microscopy.
Zhang Z, Bai L, Cong L, Yu P, Zhang T, Shi W, Li F, Du J, Wang K. Zhang Z, et al. Nat Biotechnol. 2021 Jan;39(1):74-83. doi: 10.1038/s41587-020-0628-7. Epub 2020 Aug 10. Nat Biotechnol. 2021. PMID: 32778840 - Characterizing Cortex-Wide Dynamics with Wide-Field Calcium Imaging.
Ren C, Komiyama T. Ren C, et al. J Neurosci. 2021 May 12;41(19):4160-4168. doi: 10.1523/JNEUROSCI.3003-20.2021. Epub 2021 Apr 23. J Neurosci. 2021. PMID: 33893217 Free PMC article. Review. - A Semi-supervised Pipeline for Accurate Neuron Segmentation with Fewer Ground Truth Labels.
Baker CM, Gong Y. Baker CM, et al. eNeuro. 2024 Feb 15;11(2):ENEURO.0352-23.2024. doi: 10.1523/ENEURO.0352-23.2024. Print 2024 Feb. eNeuro. 2024. PMID: 38242690 Free PMC article. - Simultaneous Optogenetics and Cellular Resolution Calcium Imaging During Active Behavior Using a Miniaturized Microscope.
Stamatakis AM, Schachter MJ, Gulati S, Zitelli KT, Malanowski S, Tajik A, Fritz C, Trulson M, Otte SL. Stamatakis AM, et al. Front Neurosci. 2018 Jul 24;12:496. doi: 10.3389/fnins.2018.00496. eCollection 2018. Front Neurosci. 2018. PMID: 30087590 Free PMC article. - Genetically encoded fluorescent sensors for imaging neuronal dynamics in vivo.
Day-Cooney J, Dalangin R, Zhong H, Mao T. Day-Cooney J, et al. J Neurochem. 2023 Feb;164(3):284-308. doi: 10.1111/jnc.15608. Epub 2022 Apr 9. J Neurochem. 2023. PMID: 35285522 Free PMC article. Review.
References
- Meltzer SJ. Emil Du Bois-Reymond. Science. 1897;5:217–219. - PubMed
- Smith SL, Judy JW, Otis TS. An ultra small array of electrodes for stimulating multiple inputs into a single neuron. Journal of Neuroscience Methods. 2004;133:109–114. - PubMed
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of Tetrode Spike Separation as Determined by Simultaneous Intracellular and Extracellular Measurements. Journal of Neurophysiology. 2000;84:401–414. - PubMed
- Nicolelis MAL, Ribeiro S. Multielectrode recordings: the next steps. Current Opinion in Neurobiology. 2002;12:602–606. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources