Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen - PubMed (original) (raw)
. 2016 Oct;22(10):1101-1107.
doi: 10.1038/nm.4184. Epub 2016 Aug 29.
Emily M Lee 3, Zhexing Wen 4 5 6 7, Yichen Cheng 3, Wei-Kai Huang 7 8, Xuyu Qian 7 9, Julia Tcw 10, Jennifer Kouznetsova 1, Sarah C Ogden 3, Christy Hammack 3, Fadi Jacob 7 11, Ha Nam Nguyen 7 12, Misha Itkin 1, Catherine Hanna 3, Paul Shinn 1, Chase Allen 3, Samuel G Michael 1, Anton Simeonov 1, Wenwei Huang 1, Kimberly M Christian 7 12, Alison Goate 10, Kristen J Brennand 13, Ruili Huang 1, Menghang Xia 1, Guo-Li Ming 7 9 11 12 14 15, Wei Zheng 1, Hongjun Song 7 9 11 12 15, Hengli Tang 3
Affiliations
- PMID: 27571349
- PMCID: PMC5386783
- DOI: 10.1038/nm.4184
Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen
Miao Xu et al. Nat Med. 2016 Oct.
Abstract
In response to the current global health emergency posed by the Zika virus (ZIKV) outbreak and its link to microcephaly and other neurological conditions, we performed a drug repurposing screen of ∼6,000 compounds that included approved drugs, clinical trial drug candidates and pharmacologically active compounds; we identified compounds that either inhibit ZIKV infection or suppress infection-induced caspase-3 activity in different neural cells. A pan-caspase inhibitor, emricasan, inhibited ZIKV-induced increases in caspase-3 activity and protected human cortical neural progenitors in both monolayer and three-dimensional organoid cultures. Ten structurally unrelated inhibitors of cyclin-dependent kinases inhibited ZIKV replication. Niclosamide, a category B anthelmintic drug approved by the US Food and Drug Administration, also inhibited ZIKV replication. Finally, combination treatments using one compound from each category (neuroprotective and antiviral) further increased protection of human neural progenitors and astrocytes from ZIKV-induced cell death. Our results demonstrate the efficacy of this screening strategy and identify lead compounds for anti-ZIKV drug development.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
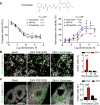
Emricasan suppresses cell death of ZIKV-infected human astrocytes and hNPCs in 2D monolayer cultures and in 3D brain organoids. (a) Top, chemical structure of Emricasan. Bottom, dose-response curves displaying the effect of Emricasan treatment on caspase activity (left) and cell viability (right) in SNB-19 glioblastoma cells exposed to 3 different ZIKV strains. Values represent mean ± s.d. (n = 3 cultures). Curves represent best fits for calculating IC50, and the insets in each panel report the calculated IC50 value against each strain. (b) Left, example images of 2D monolayer cultures of forebrain-specific hNPCs immunostained for ZIKV envelop protein (ZIKVE; green), cleaved-caspase-3 (Cas3; red) and DAPI (gray) 72 hours after ZIKV FSS-13025 exposure (with or without Emricasan treatment) compared to mock infections (multiplicity of infection/MOI = 0.04 – 0.08; scale bar: 20 μm, applies to all image panels in b). Right, quantification comparing the percentage of CAS3+ cells over DAPI in mock or ZIKV-infected hNPCs cultures treated with the indicated concentrations of Emricasan. Values represent mean ± s.e.m. (n = 3 cultures; **P < 0.01; One-way ANOVA). (c) Left, example images of 3D human forebrain-specific organoid cultures (28 days in vitro) immunostained for ZIKVE (green), Cas3 (red) and DAPI (gray), 10 days after ZIKV FSS-13025 exposure (Scale bar: 100 μm, applies to all image panels in c). Right, quantification of the percentage of CAS3+ cells over DAPI in the ventricular zone. Values represent mean ± s.e.m. (n = 6 organoids; ***P < 0.001; One-way ANOVA).
Figure 2
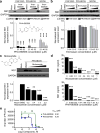
Identification of PHA-690509 and Niclosamide as antiviral compounds. (a) Top, example western blot images of titration of PHA-690509 on SNB-19 cells for anti-ZIKV activity. Glioblastoma SNB-19 cells were treated with the indicated compound for 1 hour prior to inoculation with ZIKV FSS-13025, PRVABC59, or MR766 (MOI = 1) and cells were harvested 24 hours post infection for western blot analysis. Bottom, quantification of NS1/GAPDH protein band intensities. Data were normalized to that with the DMSO treatment. Values represent mean ± s.d. (n = 3 cultures; ***P < 0.001; One-way ANOVA for comparison with the DMSO treatment). Insert shows chemical structure of PHA-690509. (**b**) _Top_, example western blot images of titration of Emricasan on SNB-19 cells for anti-ZIKV activity. Similar to (**a**). Values represent mean ± s.d. (n = 3 cultures; P > 0.1; One-way ANOVA for comparison with the DMSO treatment). (c) Top, chemical structure of Niclosamide and example western blot images of titration of Niclosamide on SNB-19 cells for anti-ZIKV activity using the PRVABC59 strain (MOI = 1). Bottom, quantification similar to (a). Values represent mean ±s.d. (n = 3 cultures; **P < 0.01; ***P < 0.001; One-way ANOVA for comparison with the DMSO treatment). (d) Summary of ELISA quantifications of secreted ZIKV-NS1 protein in the medium of infected cells upon treatment of different doses of Niclosamide (top) or PHA-690509 (bottom) for 24 hours. Values represent mean ± s.d. (n = 3 cultures; ***P < 0.001; One-way ANOVA for comparison with the 0 μM group). (e) Summary of titration of Niclosamide and PHA-690509 on ZIKV viral production. SNB-19 cells were treated with each indicated compound at increasing concentrations for 1 hour prior to infection with PRVABC59 at MOI = 0.5. Cell culture supernatant was collected 24 hours post infection and infectious virions were quantified for focus-forming units (FFU) using Vero cells. Data represent mean ± s.d. (n = 3 cultures). Curves represent best fits for calculating IC50, and the insets report the calculated IC50 value for each compound.
Figure 3
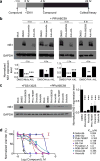
Inhibition of ZIKV infection by Niclosamide and PHA-690509 at a post-entry step and blockade of ZIKV infection by additional CDKis. (a) Schematic illustration of time-of-addition experiment for Niclosamide and PHA-690509. (b) Top, example western blot images of SNB-19 cells treated with 10 μg/ml anti-AXL antibody, 2 μM Niclosamide, or 92 μM PHA-690509 for 1 hour prior to or 4 hours post infection with PRVABC59 (MOI = 1). Bottom, quantification of NS1/GAPDH protein band intensities. Data were normalized to that with the DMSO treatment. Values represent mean ± s.d. (n = 3 cultures; *P < 0.05; **P < 0.01; ***P < 0.001; One-way ANOVA for comparison with the DMSO treatment). (c) Inhibition of ZIKV infection in CDKi-treated cells. Left, example western blot images of SNB-19 cells treated with the indicated compound at 92 μM for one hour prior to infection with FSS13025 or PRVABC59 (MOI = 1). Right, quantification similar to (b). Values represent mean ± s.d. (n = 3 cultures; ***P < 0.01; One-way ANOVA for comparison with the DMSO treatment). (d) Effect of various CDKis on ZIKV production. Similar to Figure 2e. All data were normalized to 0 μM for each compound. Data represent mean ± s.d. (n = 3 cultures). Curves represent best fits for calculating IC50 (listed on the right panel).
Figure 4
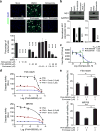
Niclosamide and PHA-690509 inhibit ZIKV infection in human astrocytes and forebrain-specific hNPCs. (a) Top, example immunostaining images of astrocytes treated with 2 μM Niclosamide, 92 μM PHA-690509, 9 μM Emricasan, or a combination of 92 μM PHA-690509 and 9 μM Emricasan for 1 hour prior to infection with PRVABC59 (MOI = 0.5). Cells were fixed 24 hours post infection and stained for ZIKVE (green) and DAPI (blue; Scale bar: 50 μm, applies to all image panels in a). Bottom, quantification of the percentage of ZIKV-infected astrocytes over DAPI. Values represent mean ± s.d. (n = 6 cultures; ***P < 0.001; One-way ANOVA for comparison with the DMSO treatment). (b) Top, example western blot images of hNPCs infected at a MOI of 0.1 and analyzed 48 hours post infection. Bottom, quantifications of NS1/GAPDH protein band intensities. Data were normalized to that with the DMSO treatment. Values represent mean ± s.d. (n = 3 cultures; ***P < 0.01; One-way ANOVA for comparison with the DMSO treatment). (c) Virus production from compound treated iPSC-derived human astrocytes. Similar to Figure 2e. Data represent mean ± s.d. (n = 3 cultures). (d–e) Summary of additive effects of a combination of Emricasan and PHA-690509 on inhibiting increased caspase-3 activity in human astrocytes infected with FSS13025-ZIKV or MR766 ZIKV (d), and on improving cell viability as measured by ATP production (e). Similar to Figure 1a. Values represent mean ± s.d. (n = 3 cultures; *P < 0.05; **P < 0.01; One-way ANOVA).
Similar articles
- High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain.
Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, Mukherjee S, Tomishima M, Brennand KJ, Zhang Q, Schwartz RE, Evans T, Studer L, Chen S. Zhou T, et al. Cell Stem Cell. 2017 Aug 3;21(2):274-283.e5. doi: 10.1016/j.stem.2017.06.017. Epub 2017 Jul 20. Cell Stem Cell. 2017. PMID: 28736217 Free PMC article. - Identification of Inhibitors of ZIKV Replication.
Morales Vasquez D, Park JG, Ávila-Pérez G, Nogales A, de la Torre JC, Almazan F, Martinez-Sobrido L. Morales Vasquez D, et al. Viruses. 2020 Sep 18;12(9):1041. doi: 10.3390/v12091041. Viruses. 2020. PMID: 32961956 Free PMC article. - The Compound SBI-0090799 Inhibits Zika Virus Infection by Blocking De Novo Formation of the Membranous Replication Compartment.
Riva L, Goellner S, Biering SB, Huang CT, Rubanov AN, Haselmann U, Warnes CM, De Jesus PD, Martin-Sancho L, Terskikh AV, Harris E, Pinkerton AB, Bartenschlager R, Chanda SK. Riva L, et al. J Virol. 2021 Oct 27;95(22):e0099621. doi: 10.1128/JVI.00996-21. Epub 2021 Sep 1. J Virol. 2021. PMID: 34468177 Free PMC article. - Strategies for Zika drug discovery.
Zou J, Shi PY. Zou J, et al. Curr Opin Virol. 2019 Apr;35:19-26. doi: 10.1016/j.coviro.2019.01.005. Epub 2019 Mar 7. Curr Opin Virol. 2019. PMID: 30852345 Review. - Recent trends in ZikV research: A step away from cure.
Alam A, Imam N, Farooqui A, Ali S, Malik MZ, Ishrat R. Alam A, et al. Biomed Pharmacother. 2017 Jul;91:1152-1159. doi: 10.1016/j.biopha.2017.05.045. Epub 2017 May 17. Biomed Pharmacother. 2017. PMID: 28531943 Review.
Cited by
- Recent advances in organoid engineering: A comprehensive review.
Unagolla JM, Jayasuriya AC. Unagolla JM, et al. Appl Mater Today. 2022 Dec;29:101582. doi: 10.1016/j.apmt.2022.101582. Epub 2022 Jul 6. Appl Mater Today. 2022. PMID: 38264423 Free PMC article. - Advanced bioinformatics rapidly identifies existing therapeutics for patients with coronavirus disease-2019 (COVID-19).
Kim J, Zhang J, Cha Y, Kolitz S, Funt J, Escalante Chong R, Barrett S, Kusko R, Zeskind B, Kaufman H. Kim J, et al. J Transl Med. 2020 Jun 25;18(1):257. doi: 10.1186/s12967-020-02430-9. J Transl Med. 2020. PMID: 32586380 Free PMC article. - Non-Invasive Quality Control of Organoid Cultures Using Mesofluidic CSTR Bioreactors and High-Content Imaging.
Charles S, Jackson-Holmes E, Sun G, Zhou Y, Siciliano B, Niu W, Han H, Nikitina A, Kemp ML, Wen Z, Lu H. Charles S, et al. bioRxiv [Preprint]. 2024 Jul 23:2024.07.19.604365. doi: 10.1101/2024.07.19.604365. bioRxiv. 2024. PMID: 39091761 Free PMC article. Preprint. - Application Progress of Organoids in Colorectal Cancer.
Luo L, Ma Y, Zheng Y, Su J, Huang G. Luo L, et al. Front Cell Dev Biol. 2022 Feb 22;10:815067. doi: 10.3389/fcell.2022.815067. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35273961 Free PMC article. Review. - Past, Present, and Future of Brain Organoid Technology.
Koo B, Choi B, Park H, Yoon KJ. Koo B, et al. Mol Cells. 2019 Sep 30;42(9):617-627. doi: 10.14348/molcells.2019.0162. Mol Cells. 2019. PMID: 31564073 Free PMC article. Review.
References
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. - PubMed
- D’Ortenzio E, et al. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. - PubMed
- McCarthy M. Zika virus was transmitted by sexual contact in Texas, health officials report. Bmj. 2016;352:i720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS097206/NS/NINDS NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- R01 MH105128/MH/NIMH NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
- U19 MH106434/MH/NIMH NIH HHS/United States
- R01 MH106056/MH/NIMH NIH HHS/United States
- R01 NS047344/NS/NINDS NIH HHS/United States
- T32 GM008752/GM/NIGMS NIH HHS/United States
- ZIA TR000018-02/ImNIH/Intramural NIH HHS/United States
- R56 MH101454/MH/NIMH NIH HHS/United States
- U01 AG046170/AG/NIA NIH HHS/United States
- T32 MH015330/MH/NIMH NIH HHS/United States
- R21 NS095348/NS/NINDS NIH HHS/United States
- ZIA TR000269-01/ImNIH/Intramural NIH HHS/United States
- R21 AI119530/AI/NIAID NIH HHS/United States
- R01 MH101454/MH/NIMH NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials