MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2 - PubMed (original) (raw)
. 2016 Feb 22;1(3):16004.
doi: 10.1038/nmicrobiol.2016.4.
Man-Lung Yeung 1 2 3 4, Lilong Jia 2, Jasper F W Chan 1 2 3 4, Kwok-Hung Chan 2, Kwok-Fan Cheung 6, Honglin Chen 1 2 3 4, Vincent K M Poon 2, Alan K L Tsang 2, Kelvin K W To 1 2 3 4, Ming-Kwong Yiu 7, Jade L L Teng 2, Hin Chu 2, Jie Zhou 1 2 3 4, Qing Zhang 6, Wei Deng 5, Susanna K P Lau 1 2 3 4, Johnson Y N Lau 4, Patrick C Y Woo 1 2 3 4, Tak-Mao Chan 6, Susan Yung 6, Bo-Jian Zheng 1 2 3 4, Dong-Yan Jin 8, Peter W Mathieson 6 9, Chuan Qin 5, Kwok-Yung Yuen 1 2 3 4
Affiliations
- PMID: 27572168
- PMCID: PMC7097571
- DOI: 10.1038/nmicrobiol.2016.4
MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2
Man-Lung Yeung et al. Nat Microbiol. 2016.
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) causes sporadic zoonotic disease and healthcare-associated outbreaks in human. MERS is often complicated by acute respiratory distress syndrome (ARDS) and multi-organ failure(1,2). The high incidence of renal failure in MERS is a unique clinical feature not often found in other human coronavirus infections(3,4). Whether MERS-CoV infects the kidney and how it triggers renal failure are not understood(5,6). Here, we demonstrated renal infection and apoptotic induction by MERS-CoV in human ex vivo organ culture and a nonhuman primate model. High-throughput analysis revealed that the cellular genes most significantly perturbed by MERS-CoV have previously been implicated in renal diseases. Furthermore, MERS-CoV induced apoptosis through upregulation of Smad7 and fibroblast growth factor 2 (FGF2) expression in both kidney and lung cells. Conversely, knockdown of Smad7 effectively inhibited MERS-CoV replication and protected cells from virus-induced cytopathic effects. We further demonstrated that hyperexpression of Smad7 or FGF2 induced a strong apoptotic response in kidney cells. Common marmosets infected by MERS-CoV developed ARDS and disseminated infection in kidneys and other organs. Smad7 and FGF2 expression were elevated in the lungs and kidneys of the infected animals. Our results provide insights into the pathogenesis of MERS-CoV and host targets for treatment.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
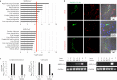
Figure 1. Ex vivo and in vitro experiments demonstrating renal infection by MERS-CoV.
a, Pathway analyses of MERS-CoV-infected (top) and SARS-CoV-infected (bottom) Calu-3 cells based on differential gene expression profiles. Red line: the most significant –log(P value) of the most perturbed biological pathway by SARS-CoV. The –log(P value) is calculated based on the hypergeometric distribution (right-tailed Fisher's exact test) of a gene of interest that can be found in a molecular network over that of a randomly selected gene. Data were derived from two independent experiments. b, Ex vivo human kidney cultures were inoculated with either MERS-CoV or SARS-CoV. The MERS-CoV-inoculated human kidneys were cryosectioned at 24 h post inoculation (top). NP of MERS-CoV was immunostained by specific antibodies (green, left). The kidneys were co-stained with cell-type-specific antibodies against cytokeratin 18 (CK18), CD31 and synaptopodin (red, middle). Co-localization of viral antigens with cellular markers is shown in yellow (right). Nuclei counterstained by DAPI are shown in blue. Representative images from three independent experiments are shown. Total RNAs of MERS-CoV-inoculated (left, lanes 1–5) and SARS-CoV-inoculated (right, lanes 1–5) human kidneys samples were collected at the indicated time points post infection (bottom). Viral RNAs were detected by RT-qPCR as described previously. MERS-CoV-infected HK2 (left, ‘+’, lane 6) and SARS-CoV-infected Calu-3 samples (right, ‘+’, lane 6) were included as positive controls. Mock-treated HK2 (left, ‘–’, lane 7) and Calu-3 (right, ‘–’, lane 7) samples were included as negative controls. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was also detected as a loading control. c, Conditioned medium (left) and cell lysate (right) of MERS-CoV-infected (M) and SARS-CoV-infected (S) HK2 and NHMC cells were collected 24 h post inoculation and the viruses were quantified using TCID50 assays as described. C, mock infected; M, MERS-CoV-infected; S, SARS-CoV-infected. Error bars represent the mean ± s.d. of three independent experiments. Samples from a–c represent biological replicates.
Figure 2. MERS-CoV infection induced the expression of caspase-3, Smad7 and FGF2.
a, Apoptosis of MERS-CoV-infected (M) and SARS-CoV-infected (S) HK2 cells were measured by fluorometric analysis of caspase-3 activity. Mock-infected cells (C) were included as negative control. a.u., arbitrary units. b, Western blot analysis of caspase-3, Smad7, FGF2, MERS-CoV NP and SARS-CoV NP in MERS-CoV-, SARS- and mock-infected HK2 cells. γ-Tubulin was also detected as a loading control. Representative images from three independent experiments are shown. c, Heat map showing the candidate genes in the category of ‘renal necrosis/cell death’ identified in Fig. 1a. Arrows point to SMAD7 and FGF2, which were selected for further study. d, The relative expression of Smad7 (left) and FGF2 (right) mRNA was measured using RT-qPCR. e, Quantitative measurement of secreted FGF2 was performed using an anti-FGF2 enzyme-linked immunosorbent assay kit. Statistical significance was evaluated by Student's _t_-tests and P values are shown in a,d,e. Except for c, all samples were harvested at 24 h.p.i. for measurements. Error bars in a,d,e represent the mean ± s.d. of three independent experiments. Samples from a–e represent biological replicates.
Figure 3. Suppression of Smad7 and FGF2 expression subverted MERS-CoV-induced apoptosis.
a, MERS-CoV-induced Smad7 expression in HK2 cells was dampened by transfection of siRNAs (Smad7-1 siRNA, lane 1; Smad7-2 siRNA, lane 2). Control siRNA-transfected (lane 3) and mock-infected (lane 4) cells were analysed. Left panels: protein expression of Smad7 and cleaved caspase-3. γ-Tubulin was detected as a loading control. Right panels: mRNA levels of Smad7 normalized to GAPDH and the relative apoptotic activities of the MERS-CoV-infected cells. b, FGF2 inhibition by a small-molecule compound or antibodies. Caspase-3 activities in MERS-CoV-infected HK2 cells were measured when FGF2 signalling was blocked with a small-molecule inhibitor AG1296 (top, lane 1). AG490 (top, lane 2), which blocks epidermal growth factor but not FGF2 signalling, and untreated MERS-CoV-infected cells (top, lane 3) served as negative and mock controls, respectively. Caspase-3 expression was determined in MERS-CoV-infected HK2 cells treated with anti-FGF2 neutralizing antibodies (bottom, lane 1) or irrelevant IgG (bottom, lane 2). Mock-infected cells were assessed (bottom, lane 3). γ-Tubulin was detected as the loading control. c, Cell protection by anti-Smad7 oligonucleotide against MERS-CoV-induced cytotoxicity in HK2 (dark diamonds) and Calu-3 (grey squares) cells when compared with that of untreated MERS-CoV infected cell lines. d, The antiviral activity of anti-Smad7 oligonucleotide was measured by comparing the levels of viral load from the MERS-CoV-infected cell extracts. All statistical significance was evaluated by Student's _t_-tests (P) and Pearson's correlation analyses (r). Images shown in a and b are representative of three independent experiments. Error bars in a–d represent the mean ± s.d. of three independent experiments. Samples from a–d represent biological replicates.
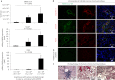
Figure 4. Viral loads and host gene expression in the lung and kidney of common marmosets inoculated with MERS-CoV on day 3 post infection.
a, Quantitative measurement of MERS-CoV RNA in the lungs of MERS-CoV-inoculated common marmosets. Samples were divided into three categories (1.9 × 105 ± 9.9 × 104; 1.6 × 106 ± 5.2 × 105; 2.6 × 106 ± 1.7 × 106) based on the viral load (top). The relative expression levels of Smad7 (middle) and FGF2 (bottom) are indicated. All values were normalized to GAPDH. Statistical significance was evaluated by Student's _t_-tests and P values are indicated. Error bars represent the mean ± s.d. of three selected tissue samples. b, Co-immunohistochemical staining of MERS-CoV NP, caspase-3, Smad7 and FGF2 in kidneys of MERS-CoV-inoculated common marmosets. All co-immunohistochemical staining was performed on the same slide, except Smad7 and MERS-CoV NP, which were stained on separate slides, and the overlay view was generated using two neighbouring slides. The specificity of anti-MERS-CoV antibodies was confirmed by overnight pre-incubation with a fivefold more concentrated MERS-CoV NP peptide before their application to the kidney sections. The central image in the bottom panel represents the background signal detected after inoculation of rabbit secondary antibodies. Nuclei counterstained by DAPI are shown in blue. c, Haematoxylin and eosin staining of kidney sections of MERS-CoV-inoculated common marmosets. Interstitial infiltration was observed in the infected kidneys (arrow heads). Characteristic histological features of acute kidney injury, including flattened epithelial cells (arrows) and peritubular capillary congestion (white arrows), were detected in the kidney sections of MERS-CoV-inoculated common marmosets. Images shown in b and c are representatives of the four common marmosets' kidneys in which the MERS-CoV RNAs were detected (Supplementary Fig. 8a). D, dilated renal tubules; PC, protein cast.
Similar articles
- CD8+ T Cells and Macrophages Regulate Pathogenesis in a Mouse Model of Middle East Respiratory Syndrome.
Coleman CM, Sisk JM, Halasz G, Zhong J, Beck SE, Matthews KL, Venkataraman T, Rajagopalan S, Kyratsous CA, Frieman MB. Coleman CM, et al. J Virol. 2016 Dec 16;91(1):e01825-16. doi: 10.1128/JVI.01825-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795435 Free PMC article. - Acute Respiratory Infection in Human Dipeptidyl Peptidase 4-Transgenic Mice Infected with Middle East Respiratory Syndrome Coronavirus.
Iwata-Yoshikawa N, Okamura T, Shimizu Y, Kotani O, Sato H, Sekimukai H, Fukushi S, Suzuki T, Sato Y, Takeda M, Tashiro M, Hasegawa H, Nagata N. Iwata-Yoshikawa N, et al. J Virol. 2019 Mar 5;93(6):e01818-18. doi: 10.1128/JVI.01818-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626685 Free PMC article. - An Acute Immune Response to Middle East Respiratory Syndrome Coronavirus Replication Contributes to Viral Pathogenicity.
Baseler LJ, Falzarano D, Scott DP, Rosenke R, Thomas T, Munster VJ, Feldmann H, de Wit E. Baseler LJ, et al. Am J Pathol. 2016 Mar;186(3):630-8. doi: 10.1016/j.ajpath.2015.10.025. Epub 2015 Dec 24. Am J Pathol. 2016. PMID: 26724387 Free PMC article. - A Comparative Review of Animal Models of Middle East Respiratory Syndrome Coronavirus Infection.
Baseler L, de Wit E, Feldmann H. Baseler L, et al. Vet Pathol. 2016 May;53(3):521-31. doi: 10.1177/0300985815620845. Epub 2016 Feb 11. Vet Pathol. 2016. PMID: 26869154 Review. - Modulation of the immune response by Middle East respiratory syndrome coronavirus.
Shokri S, Mahmoudvand S, Taherkhani R, Farshadpour F. Shokri S, et al. J Cell Physiol. 2019 Mar;234(3):2143-2151. doi: 10.1002/jcp.27155. Epub 2018 Aug 26. J Cell Physiol. 2019. PMID: 30146782 Free PMC article. Review.
Cited by
- Emerging fatal gout disease in Chinese goslings linked to acute kidney injury induced by novel goose astrovirus infection.
Bi Z, Lv X, Zhang Z, Cai L, Zhang M, Li W, Ding Y, Liu H, Yang K, Zhu Y, Liu G, Wang G. Bi Z, et al. Front Cell Infect Microbiol. 2024 Sep 18;14:1470808. doi: 10.3389/fcimb.2024.1470808. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39359936 Free PMC article. - A glimpse into viral warfare: decoding the intriguing role of highly pathogenic coronavirus proteins in apoptosis regulation.
Cheng L, Rui Y, Wang Y, Chen S, Su J, Yu XF. Cheng L, et al. J Biomed Sci. 2024 Jul 13;31(1):70. doi: 10.1186/s12929-024-01062-1. J Biomed Sci. 2024. PMID: 39003473 Free PMC article. Review. - MPASL: multi-perspective learning knowledge graph attention network for synthetic lethality prediction in human cancer.
Zhang G, Chen Y, Yan C, Wang J, Liang W, Luo J, Luo H. Zhang G, et al. Front Pharmacol. 2024 May 21;15:1398231. doi: 10.3389/fphar.2024.1398231. eCollection 2024. Front Pharmacol. 2024. PMID: 38835667 Free PMC article. - Kidney Function Tests and Continuous eGFR Decrease at Six Months after SARS-CoV-2 Infection in Patients Clinically Diagnosed with Post-COVID Syndrome.
Boruga M, Septimiu-Radu S, Nandarge PS, Elagez A, Doros G, Lazureanu VE, Stoicescu ER, Tanase E, Iacob R, Dumitrescu A, Bota AV, Cotoraci C, Bratu ML. Boruga M, et al. Biomedicines. 2024 Apr 24;12(5):950. doi: 10.3390/biomedicines12050950. Biomedicines. 2024. PMID: 38790912 Free PMC article. - Mitochondria protective and anti-apoptotic effects of peripheral benzodiazepine receptor and its ligands on the treatment of asthma in vitro and vivo.
Liu Y, Zhang Z, He Y, Li R, Zhang Y, Liu H, Wang Y, Ma W. Liu Y, et al. J Inflamm (Lond). 2024 Apr 19;21(1):11. doi: 10.1186/s12950-024-00383-0. J Inflamm (Lond). 2024. PMID: 38641850 Free PMC article.
References
- Eckerle I, Muller MA, Kallies S, Gotthardt DN, Drosten C. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East Respiratory Syndrome (MERS) Coronavirus infection. Virol. J. 2013;10,:359. doi: 10.1186/1743-422X-10-359. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources