Phase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancer - PubMed (original) (raw)
Clinical Trial
. 2016 Sep 27;7(39):64089-64099.
doi: 10.18632/oncotarget.11596.
Andrea L A Wong 1 2 3, Ting-Ting Wang 3, Thian-C Ng 4, Bo Zhang 4, Sing-Huang Tan 1 2, Thomas I P Soh 1 2, Angela S L Pang 1 2, Chee-Seng Tan 1 2, Samuel G W Ow 1 2, Lingzhi Wang 3 5, Jannet Mogro 2, Jingshan Ho 1 2, Anand D Jeyasekharan 1 2 3, Yiqing Huang 1 2, Choon-Hua Thng 6, Ching-Wan Chan 7, Mikael Hartman 7, Philip Iau 7, Shaik A Buhari 7, Boon-Cher Goh 1 2 3 5, Soo-Chin Lee 1 2 3
Affiliations
- PMID: 27577069
- PMCID: PMC5325427
- DOI: 10.18632/oncotarget.11596
Clinical Trial
Phase Ib/II randomized, open-label study of doxorubicin and cyclophosphamide with or without low-dose, short-course sunitinib in the pre-operative treatment of breast cancer
Andrea L A Wong et al. Oncotarget. 2016.
Abstract
Background: Prolonged anti-angiogenic therapy destroys tumor vasculature, whereas vascular-normalizing doses may enhance intra-tumoral drug delivery. We hypothesize that low-dose, short-course sunitinib normalizes vasculature, enhancing chemotherapy efficacy.
Patients and methods: In phase Ib, treatment-naïve breast cancer patients received four cycles of pre-operative doxorubicin/cyclophosphamide, with sunitinib before each cycle. The optimal dose of sunitinib leading to tumor vessel normalization on immunohistochemistry was identified. In phase II, subjects were randomized to chemotherapy alone or chemotherapy plus sunitinib at the recommended phase II dose (RP2D). Primary endpoint was pathological complete response (pCR) rate. Tumor and functional imaging biomarkers were evaluated serially.
Results: In phase Ib (n=9), sunitinib 12.5 mg daily for 7 days before each chemotherapy was established as RP2D. In phase II, patients receiving chemotherapy plus sunitinib (n=24) had similar pCR rates (5.0% versus 4.3%, p=1.00), but a higher incidence of chemotherapy dose delays (33.3% versus 8.7%, p=0.04), compared to those receiving chemotherapy alone (n=25). The addition of sunitinib to chemotherapy significantly increased vascular normalization index (VNI) and decreased lymphatic vessel density (D2-40) on immunohistochemistry [VNI:25.50±27.94% versus 49.29±31.84%, p=0.034; D2-40:3.29±2.70 versus 1.29±1.54, p=0.014, baseline versus post-cycle 1], and improved perfusion on DCE-MRI (Ktrans:12.6±9.6 mL/100 g/min versus 16.3±10.7 mL/100 g/min, baseline versus post-cycle 1, p=0.015). Conversely, immunohistochemical and DCE-MRI parameters were not significantly altered by chemotherapy alone.
Conclusion: Low-dose, short-course sunitinib prior to anthracycline-based chemotherapy in breast cancer patients did not improve pCR and increased chemotherapy dose delays. However, the addition of sunitinib induced compelling pharmacodynamic evidence of vascular normalization. Further studies with alternative cytotoxic regimens should be explored.
Keywords: anti-angiogenic therapy; breast cancer; neoadjuvant chemotherapy; sunitinib; vascular normalization.
Conflict of interest statement
CONFLICTS OF INTEREST
All authors express no conflicts of interest in contributing to this work. This work has previously been presented in part [J Clin Oncol 30; 2012 (suppl, abstr 1064) and J Clin Oncol 32: 5s, 2014 (suppl, abstr 1060)].
Figures
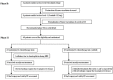
Figure 1. CONSORT diagram: trial profile
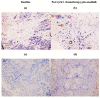
Figure 2. Immunohistochemistry staining was performed at baseline and after one cycle of chemotherapy plus sunitinib in a representative patient (200x magnification)
Compared with immunoreactivity levels in baseline tumors a. and c., chemotherapy plus sunitinib led to increased vascular normalization index [ratio of α-SMA(brown)/CD31(red)] b. and decreased lymphatic vessel density as determined by D2-40 d.
Similar articles
- Immunohistochemistry study of tumor vascular normalization and anti-angiogenic effects of sunitinib versus bevacizumab prior to dose-dense doxorubicin/cyclophosphamide chemotherapy in HER2-negative breast cancer.
Yadav K, Lim J, Choo J, Ow SGW, Wong A, Lee M, Chan CW, Hartman M, Lim SE, Ngoi N, Tang SW, Ang Y, Chan G, Chong WQ, Tan HL, Tan SH, Goh BC, Lee SC. Yadav K, et al. Breast Cancer Res Treat. 2022 Feb;192(1):131-142. doi: 10.1007/s10549-021-06470-7. Epub 2021 Dec 20. Breast Cancer Res Treat. 2022. PMID: 34928481 Free PMC article. Clinical Trial. - Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma.
Grivas PD, Daignault S, Tagawa ST, Nanus DM, Stadler WM, Dreicer R, Kohli M, Petrylak DP, Vaughn DJ, Bylow KA, Wong SG, Sottnik JL, Keller ET, Al-Hawary M, Smith DC, Hussain M. Grivas PD, et al. Cancer. 2014 Mar 1;120(5):692-701. doi: 10.1002/cncr.28477. Epub 2013 Nov 18. Cancer. 2014. PMID: 24249435 Clinical Trial. - Prediction of response to repeat utilization of anthracycline in recurrent breast cancer patients previously administered anthracycline-containing chemotherapeutic regimens as neoadjuvant or adjuvant chemotherapy.
Yonemori K, Katsumata N, Kaneko M, Uno H, Matsumoto K, Kouno T, Shimizu C, Ando M, Takeuchi M, Fujiwara Y. Yonemori K, et al. Breast Cancer Res Treat. 2007 Jul;103(3):313-8. doi: 10.1007/s10549-006-9384-8. Epub 2006 Oct 25. Breast Cancer Res Treat. 2007. PMID: 17063267 - The impact of chemotherapy dose density and dose intensity on breast cancer outcome: what have we learned?
Piccart MJ, Biganzoli L, Di Leo A. Piccart MJ, et al. Eur J Cancer. 2000 Apr;36 Suppl 1:S4-10. doi: 10.1016/s0959-8049(99)00256-7. Eur J Cancer. 2000. PMID: 10785603 Review. - Metronomic therapy and breast cancer: a systematic review.
Montagna E, Cancello G, Dellapasqua S, Munzone E, Colleoni M. Montagna E, et al. Cancer Treat Rev. 2014 Sep;40(8):942-50. doi: 10.1016/j.ctrv.2014.06.002. Epub 2014 Jun 18. Cancer Treat Rev. 2014. PMID: 24998489 Review.
Cited by
- A Phase II Study Evaluating the Safety and Efficacy of Sunitinib Malate in Combination With Weekly Paclitaxel Followed by Doxorubicin and Daily Oral Cyclophosphamide Plus G-CSF as Neoadjuvant Chemotherapy for Locally Advanced or Inflammatory Breast Cancer.
Symonds L, Jenkins I, Linden HM, Kurland B, Gralow JR, Gadi VVK, Ellis GK, Wu Q, Rodler E, Chalasani P, Chai X, Riedel J, Scca Network Investigators, Stopeck A, Brown-Glaberman U, Specht JM. Symonds L, et al. Clin Breast Cancer. 2022 Jan;22(1):32-42. doi: 10.1016/j.clbc.2021.05.009. Epub 2021 May 24. Clin Breast Cancer. 2022. PMID: 34158245 Free PMC article. Clinical Trial. - Neoadjuvant sunitinib plus exemestane in post-menopausal women with hormone receptor-positive/HER2-negative early-stage breast cancer (SUT_EXE-08): a phase I/II trial.
Fullana B, Morales S, Petit A, Alay A, Verdaguer H, Climent F, Navarro-Perez V, Cejuela M, Galvan P, Gumà A, Llombart-Cussac A, Cordero D, Casanovas O, Prat A, Gil-Gil M, Pernas S. Fullana B, et al. Sci Rep. 2024 Oct 9;14(1):23626. doi: 10.1038/s41598-024-72152-1. Sci Rep. 2024. PMID: 39384801 Free PMC article. Clinical Trial. - Immunohistochemistry study of tumor vascular normalization and anti-angiogenic effects of sunitinib versus bevacizumab prior to dose-dense doxorubicin/cyclophosphamide chemotherapy in HER2-negative breast cancer.
Yadav K, Lim J, Choo J, Ow SGW, Wong A, Lee M, Chan CW, Hartman M, Lim SE, Ngoi N, Tang SW, Ang Y, Chan G, Chong WQ, Tan HL, Tan SH, Goh BC, Lee SC. Yadav K, et al. Breast Cancer Res Treat. 2022 Feb;192(1):131-142. doi: 10.1007/s10549-021-06470-7. Epub 2021 Dec 20. Breast Cancer Res Treat. 2022. PMID: 34928481 Free PMC article. Clinical Trial. - Transport of drugs from blood vessels to tumour tissue.
Dewhirst MW, Secomb TW. Dewhirst MW, et al. Nat Rev Cancer. 2017 Dec;17(12):738-750. doi: 10.1038/nrc.2017.93. Epub 2017 Nov 10. Nat Rev Cancer. 2017. PMID: 29123246 Free PMC article. Review. - Lymphatic vessel: origin, heterogeneity, biological functions, and therapeutic targets.
Hu Z, Zhao X, Wu Z, Qu B, Yuan M, Xing Y, Song Y, Wang Z. Hu Z, et al. Signal Transduct Target Ther. 2024 Jan 3;9(1):9. doi: 10.1038/s41392-023-01723-x. Signal Transduct Target Ther. 2024. PMID: 38172098 Free PMC article. Review.
References
- Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N, National Surgical Adjuvant B, Bowel Project Protocol B The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–4174. - PubMed
- von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Kaufmann M, et al. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized GeparTrio trial. J Natl Cancer Inst. 2008;100:542–551. - PubMed
- Rugo HS. Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning? J Clin Oncol. 2012;30:898–901. - PubMed
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical