Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules - PubMed (original) (raw)
Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules
Klara Frydryskova et al. BMC Mol Biol. 2016.
Abstract
Background: Eukaryotic translation initiation factor 4E (eIF4E) plays a pivotal role in the control of cap-dependent translation initiation, modulates the fate of specific mRNAs, occurs in processing bodies (PBs) and is required for formation of stress granules (SGs). In this study, we focused on the subcellular localization of a representative compendium of eIF4E protein isoforms, particularly on the less studied members of the human eIF4E protein family, eIF4E2 and eIF4E3.
Results: We showed that unlike eIF4E1, its less studied isoform eIF4E3_A, encoded by human chromosome 3, localized to stress granules but not PBs upon both heat shock and arsenite stress. Furthermore, we found that eIF4E3_A interacts with human translation initiation factors eIF4G1, eIF4G3 and PABP1 in vivo and sediments into the same fractions as canonical eIF4E1 during polysome analysis in sucrose gradients. Contrary to this finding, the truncated human eIF4E3 isoform, eIF4E3_B, showed no localization to SGs and no binding to eIF4G. We also highlighted that eIF4E2 may exhibit distinct functions under different stresses as it readily localizes to P-bodies during arsenite and heat stresses, whereas it is redirected to stress granules only upon heat shock. We extended our study to a number of protein variants, arising from alternative mRNA splicing, of each of the three eIF4E isoforms. Our results surprisingly uncovered differences in the ability of eIF4E1_1 and eIF4E1_3 to form stress granules in response to cellular stresses.
Conclusion: Our comparison of all three human eIF4E isoforms and their protein variants enriches the intriguing spectrum of roles attributed to the eukaryotic initiation translation factors of the 4E family, which exhibit a distinctive localization within different RNA granules under different stresses. The localization of eIF4E3_A to stress granules, but not to processing bodies, along with its binding to eIF4G and PABP1 suggests a role of human eIF4E3_A in translation initiation rather than its involvement in a translational repression and mRNA decay and turnover. The localization of eIF4E2 to stress granules under heat shock but not arsenite stress indicates its distinct function in cellular response to these stresses and points to the variable protein content of SGs as a consequence of different stress insults.
Keywords: Arsenite; Eukaryotic translation initiation factor 4E (eIF4E); Heat shock; PB; Processing body (P-body); SG; Stress granule; Translation control; Translation initiation factor; eIF4E2; eIF4E3.
Figures
Fig. 1
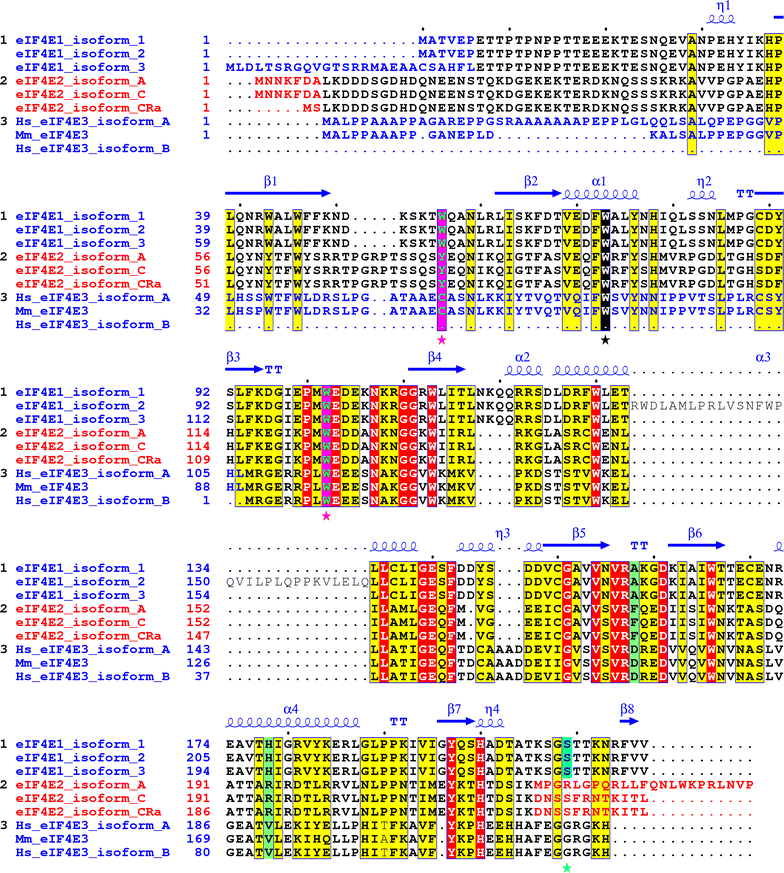
Alignment of the human eIF4E1, eIF4E2 and eIF4E3 isoforms and their variants explored in this study. Yellow and red boxes denote amino acids high similarity and identity, respectively. Utilization of alternative exons coding for different N- and C-protein termini is marked with blue and red letters. Cap-binding residues W56 and W102 in eIF4E1 and corresponding amino acids in eIF4E2 and eIF4E3 are highlighted as green letters in purple boxes and marked with purple asterisks. The conserved W73 (on the basis of eIF4E1_1) is marked with black box and black asterisk. Ser209 in eIF4E1 (numbering as of eIF4E1_1) is shaded in turquoise blue. Mouse eIF4E3 was added to highlight differences in primary structure between mouse and human orthologs. PDB file 3AM7 was used to depict eIF4E1 secondary structure [56]
Fig. 2
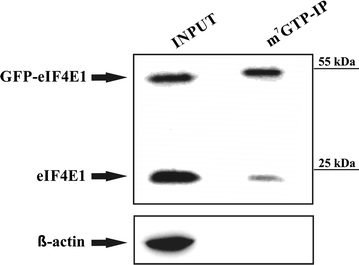
GFP-eIF4E1 fusion protein is capable to bind to m7GTP agarose. HEK293 cells were lysed (INPUT) and the lysate was incubated with the m7GTP-agarose. Western blot was developed with anti-eIF4E1 antibody, which clearly shows that both endogenous eIF4E1 and its GFP-eIF4E1 fusion counterpart retain their ability to bind the m7G cap. Actin was used as a control of a sufficient washing of the m7GTP affinity resin
Fig. 3
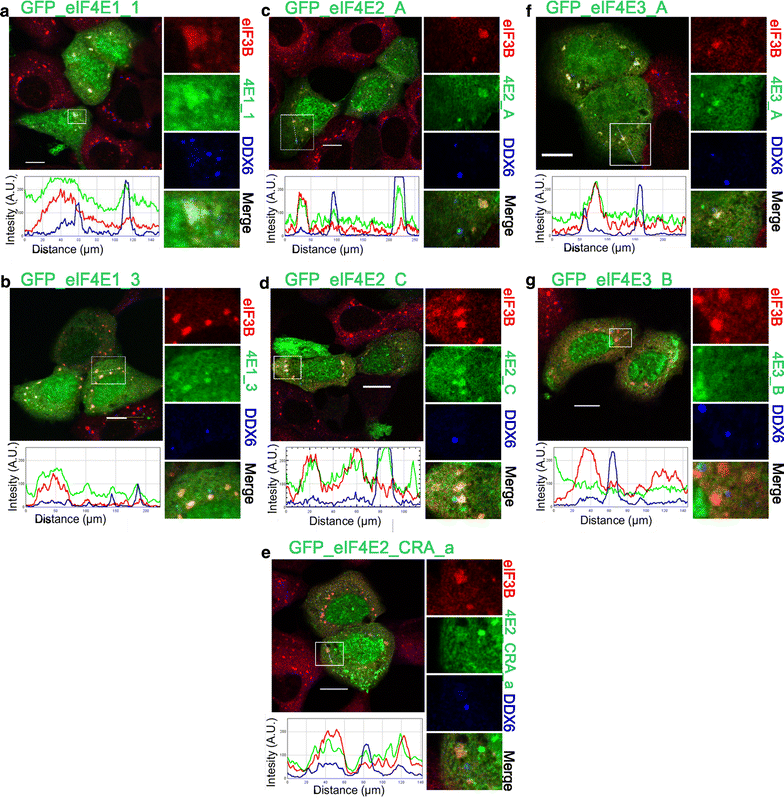
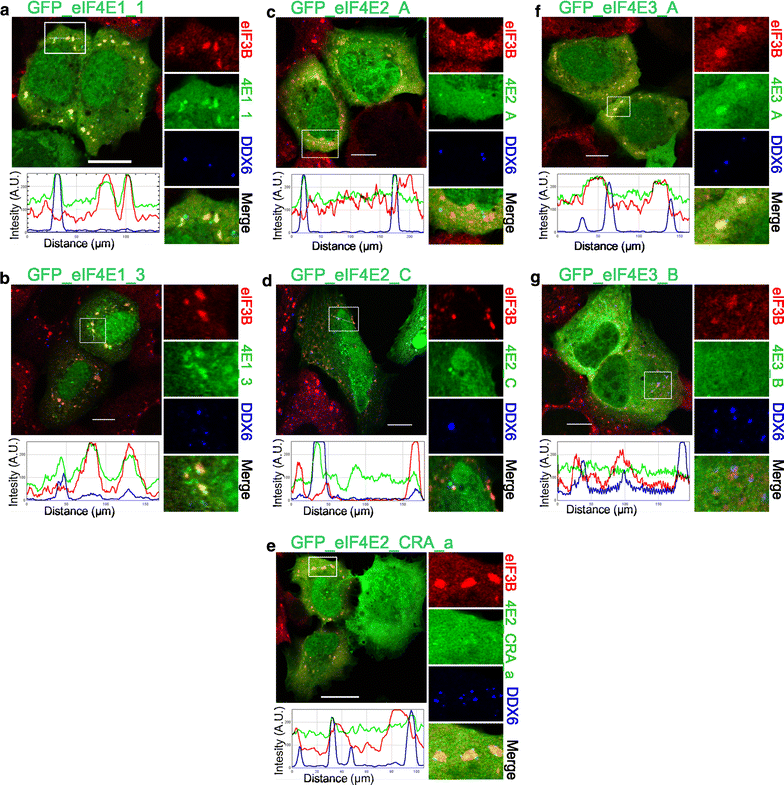
Co-localization of the eIF4E isoforms with PBs and SGs during heat shock. The eIF4E1, 2, 3 proteins (green) were ectopically produced in fusion with GFP in U2OS cells. Nineteen hours after transfection, the cells were exposed to 41.7 °C for 30 min, fixed and assessed for eIF3B-stained SGs (red) and DDX6-stained PBs (blue). Co-localization of the particular eIF4E with SGs and PBs is demonstrated in the boxed area replicated in higher magnification on the right side of each panel and by the intensity profile measured along the dashed white line within the boxed area. Both eIF4E1 (a, b) and all three eIF4E2 (c– e) variants co-localized with SGs and PBs. The eIF4E3_A (f) was recruited only to SGs, and eIF4E3_B (g) co-localized with neither SGs nor PBs. Approximately 50 cells transfected with either vector were observed in two independent biological replicates. Scale bar, 20 µm
Fig. 4
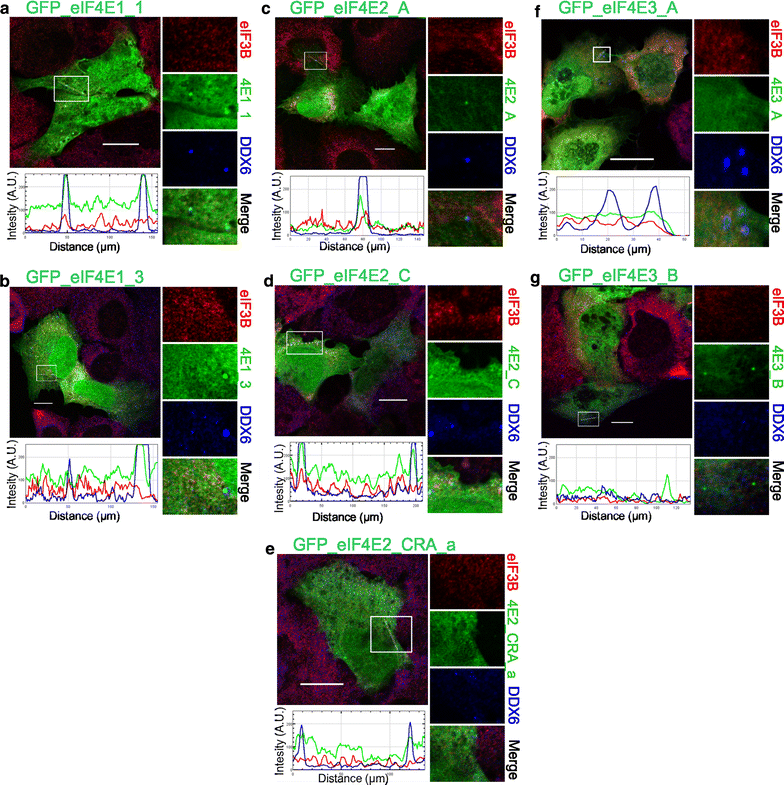
Co-localization of the eIF4E isoforms with PBs in control stress-free cells. The eIF4E1, 2, 3 proteins (green) were ectopically produced in fusion with GFP in U2OS cells. Nineteen hours after transfection, the cells were fixed and assessed for eIF3B-stained SGs (red) and DDX6-stained PBs (blue). No development of stress granules was observed. Co-localization of the particular eIF4E with PBs is demonstrated in the boxed area replicated in higher magnification on the right side of each panel and by the intensity profile measured along the dashed white line within the boxed area. Both eIF4E1 (a, b) and all three eIF4E2 (c–e) variants co-localized with PBs. No co-localization with PBs was detected for eIF4E3_A (f) or eIF4E3_B (g). Approximately 50 cells transfected with each plasmid were investigated in two independent biological replicates. Scale bar, 20 µm
Fig. 5
Co-localization of the eIF4E proteins and their isoforms with PBs and SGs during oxidative stress. The eIF4E1, 2, 3 proteins (green) were ectopically produced in fusion with GFP in U2OS cells. Nineteen hours post-transfection, the cells were treated with 1 mM sodium arsenite for 40 min, fixed and assessed for eIF3B-stained SGs (red) and DDX6-stained PBs (blue). Co-localization of the particular eIF4E with SGs and PBs is demonstrated in the boxed area on the right side of each panel and by the intensity profile measured along the dashed white line within the boxed area. Contrary to heat shock, only the eIF4E1 variants were able to co-localize with both SGs and PBs (a, b). The eIF4E2 protein variants (c–e) co-localized only with PBs. eIF4E3_A (f) was present only in SGs, and eIF4E3_B (g) co-localized with neither SGs nor PBs. Approximately 50 cells transfected with either vector were observed in two independent biological replicates. Scale bar, 20 µm
Fig. 6
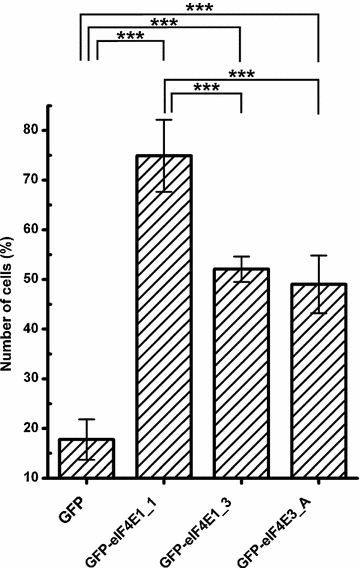
eIF4E1_3 and eIF4E3_A isoforms are less prone to form SGs than the prototypical eIF4E1_1. eIF4E1_1, eIF4E1_3, and eIF4E3_A proteins were ectopically produced in fusion with GFP from the same vector in U2OS cells. Nineteen hours post-transfection, the cells were treated with 1 mM sodium arsenite for 40 min, and those forming SGs were counted and plotted as a fraction of all transfected cells. Error bars indicate differences among three independent experiments, in which approximately 100 of the transfected cells were assessed. We applied Chi square test to analyze differences between number of cells forming stress granules among all transfected cells expressing individual eIF4E proteins or a GFP control. GFP-eIF4E1_1, GFP-eIF4E1_3, GFP-eIF4E3_A were compared to control pGFP and to each other by post hoc Chi square test with Bonferroni correction for multiple testing. Exept the GFP-eIF4E1_3 x GFP-eIF4E3_A pair, all other differences were statistically significant (p values <0.0001, marked with asterisk)
Fig. 7
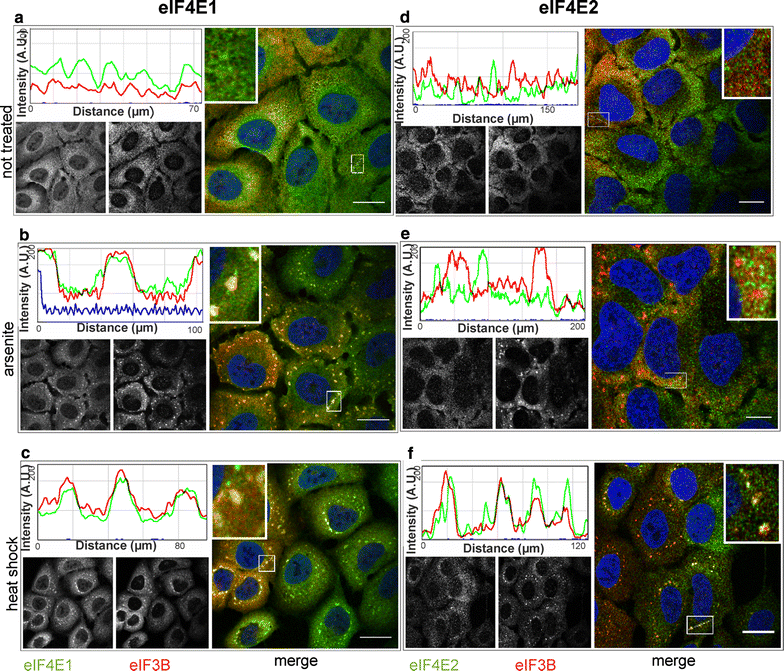
eIF4E2 becomes a component of SGs during heat shock but not in a arsenite stress. U2OS cells were grown in stress-free conditions (a, d), treated with sodium arsenite (B, E) or exposed to heat (C, F), and then stained with antibodies against eIF4E1 (a–c, green), eIF4E2 (d–f, green) and eIF3B (SG marker, red). Co-localization of the particular eIF4E with SGs is demonstrated by merge (on the right of each panel) and the intensity profile along the dashed white line in the boxed area (shown again in 3× magnification on the right side of the corresponding intensity profile). In agreement with experiments based on GFP-tagged proteins, the immunostaining of endogenous eIF4E1 and IF4E2 shows a specific recruitment of eIF4E2 to SGs during heat stress (f). Left B/W image in each panel shows localization of the corresponding endogenous eIF4E protein, right B/W image in each panel shows eIF3B. Noticeable fractions of both eIF4E1 and eIF4E2 are visibly localized in the cellular nuclei. Nuclei were stained with DAPI (blue). Approximately 50 cells were observed in each parallel. Scale bar, 20 µm
Fig. 8
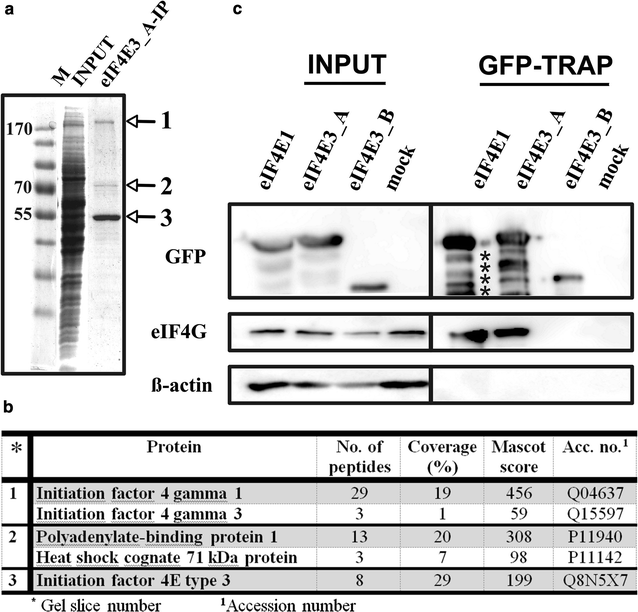
eIF4G interacts with eIF4E3_A but not with eIF4E3_B. a Coomassie blue stained gel demonstrating immunoprecipitation of the ectopically expressed GFP-eIF4E3_A from the HEK293 cell lysate using a GFP-Trap approach. M PageRuler™ Prestained Ladder (Thermo Scientific); INPUT whole cell lysate; eIF4E3_A-IP proteins co-immunoprecipitating with GFP-eIF4E3_A. b MS analysis of the proteins co-immunoprecipitating with eIF4E3_A. Gel slices are numbered as in panel A. c Western blots of proteins co-immunoprecipitating with eIF4E1_1, eIF4E3_A and eIF4E3_B transiently expressed in GFP fusion in HEK293 cells using GFP-Trap agarose beads (GFP-TRAP). Membranes were developed with anti-GFP (detecting eIF4E-GFP fusion proteins), anti-eIF4G and anti-β-actin antibodies. INPUT lines including β-actin and eIF4G served as a loading control. Lysate from non-transfected HEK293 cells was used as a negative control (mock). The absence of detectable β-actin on GFP-Trap beads shows no contamination of non-specifically bound proteins in the samples
Fig. 9
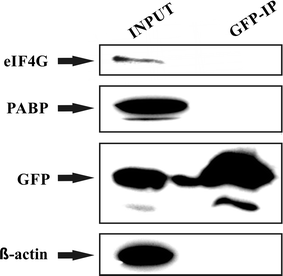
Control immunoprecipitation experiment does not reveal any non-specific interaction between GFP and eIF4G or PABP: HEK293 cells transiently transfected with a control expression vector pEGFP-C1 were lysed 24 h post-transfection and the lysate was subjected to immunoprecipitation using GFP-Trap approach. Western blots were developed with anti-eIF4G, anti-PABP, anti-GFP and anti β-actin antibodies. The results clearly show that while all the proteins tested were present in the lysate, only GFP remained bound to the resin upon GFP-Trap immunoprecipitation
Fig. 10
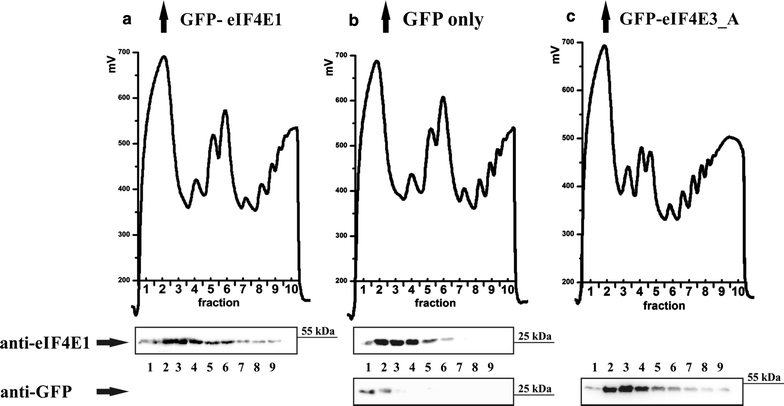
Polysome profile analysis revealed that eIF4E3_A associates with translation intiation complexes and light polysome fractions. GFP-eIF4E1 (a) and GFP-eIF4E3_A (c) stably expressed in HEK293 cells are distributed along polysome profiles similarly to endogenous eIF4E1 (b). To evaluate possible influence of a GFP-fusion tag, a polysome profile analysis from HEK293 cells stably expressing GFP alone was performed (b). Western blots of the first nine fractions were probed either with anti-eIF4E1 antibody (a, b) or anti-GFP antibody (b, c). Highest amounts of GFP-eIF4E1, GFP-eIF4E3_A and endogenous eIF4E1 were detected from the end of the loading peak to the light polysomes, whereas GFP protein alone was detected in the early loading peak exclusively. Loading peak (≈fractions 1–3); 40S, 60S and 80S (≈fractions 4–6 in a, b and 3–5 in c); light and heavy polysomes (≈fractions 7–10 in a, b and 6–10 in c)
Fig. 11
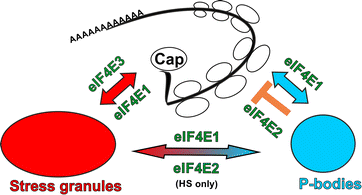
Possible roles of eIF4E1, eIF4E2 and eIF4E3 in translation inititation and mRNA repression. Abundant and tightly regulated eIF4E1 plays an important role both in translation initiation and translation repression and therefore localizes to sites of active translation, PBs and SGs. The major role of eIF4E2 is in translation repression and therefore localizes mainly to PBs. Different composition of SGs as a consequence of different stresses and dynamic flux of molecules between PBs and SGs is suggested by the presence of eIF4E2 in SGs after heat shock but not sodium arsenite treatment. Low abundant eIF4E3_A may serve as a keeper of basal translation initiation which is not regulated by 4E-BP pathway and is not involved in mRNA repression and decay pathways. eIF4E3_A thus localizes to SGs but never to PBs upon stresses. Colour coding is the same as in other figures: eIF4Es are in green, PBs are in blue and SGs are in red. For simplification, we do not include other eIF4E1 regulatory pathways and shuttling of all three eIF4Es between cytoplasm and nucleus
Similar articles
- Major splice variants and multiple polyadenylation site utilization in mRNAs encoding human translation initiation factors eIF4E1 and eIF4E3 regulate the translational regulators?
Mrvová S, Frydrýšková K, Pospíšek M, Vopálenský V, Mašek T. Mrvová S, et al. Mol Genet Genomics. 2018 Feb;293(1):167-186. doi: 10.1007/s00438-017-1375-4. Epub 2017 Sep 23. Mol Genet Genomics. 2018. PMID: 28942592 - Investigating the consequences of eIF4E2 (4EHP) interaction with 4E-transporter on its cellular distribution in HeLa cells.
Kubacka D, Kamenska A, Broomhead H, Minshall N, Darzynkiewicz E, Standart N. Kubacka D, et al. PLoS One. 2013 Aug 21;8(8):e72761. doi: 10.1371/journal.pone.0072761. eCollection 2013. PLoS One. 2013. PMID: 23991149 Free PMC article. - Two related trypanosomatid eIF4G homologues have functional differences compatible with distinct roles during translation initiation.
Moura DM, Reis CR, Xavier CC, da Costa Lima TD, Lima RP, Carrington M, de Melo Neto OP. Moura DM, et al. RNA Biol. 2015;12(3):305-19. doi: 10.1080/15476286.2015.1017233. RNA Biol. 2015. PMID: 25826663 Free PMC article. - Taking a re-look at cap-binding signatures of the mRNA cap-binding protein eIF4E orthologues in trypanosomatids.
Das S. Das S. Mol Cell Biochem. 2021 Feb;476(2):1037-1049. doi: 10.1007/s11010-020-03970-w. Epub 2020 Nov 10. Mol Cell Biochem. 2021. PMID: 33169189 Review. - Eukaryotic initiation factor 4E (eIF4E): A recap of the cap-binding protein.
Batool A, Aashaq S, Andrabi KI. Batool A, et al. J Cell Biochem. 2019 Sep;120(9):14201-14212. doi: 10.1002/jcb.28851. Epub 2019 May 9. J Cell Biochem. 2019. PMID: 31074051 Review.
Cited by
- eIF4EHP promotes Ldh mRNA translation in and fruit fly adaptation to hypoxia.
Liang M, Hody C, Yammine V, Soin R, Sun Y, Lin X, Tian X, Meurs R, Perdrau C, Delacourt N, Oumalis M, Andris F, Conrard L, Kruys V, Gueydan C. Liang M, et al. EMBO Rep. 2023 Jul 5;24(7):e56460. doi: 10.15252/embr.202256460. Epub 2023 May 5. EMBO Rep. 2023. PMID: 37144276 Free PMC article. - eIF4E3 forms an active eIF4F complex during stresses (eIF4FS) targeting mTOR and re-programs the translatome.
Weiss B, Allen GE, Kloehn J, Abid K, Jaquier-Gubler P, Curran JA. Weiss B, et al. Nucleic Acids Res. 2021 May 21;49(9):5159-5176. doi: 10.1093/nar/gkab267. Nucleic Acids Res. 2021. PMID: 33893802 Free PMC article. - Translation Initiation Factor eIF4E Positively Modulates Conidiogenesis, Appressorium Formation, Host Invasion and Stress Homeostasis in the Filamentous Fungi Magnaporthe oryzae.
Batool W, Shabbir A, Lin L, Chen X, An Q, He X, Pan S, Chen S, Chen Q, Wang Z, Norvienyeku J. Batool W, et al. Front Plant Sci. 2021 Jun 16;12:646343. doi: 10.3389/fpls.2021.646343. eCollection 2021. Front Plant Sci. 2021. PMID: 34220879 Free PMC article. - Stress Granules as Causes and Consequences of Translation Suppression.
Baymiller M, Moon SL. Baymiller M, et al. Antioxid Redox Signal. 2023 Aug;39(4-6):390-409. doi: 10.1089/ars.2022.0164. Epub 2023 Jun 28. Antioxid Redox Signal. 2023. PMID: 37183403 Free PMC article. Review. - Post-transcriptional control of gene expression following stress: the role of RNA-binding proteins.
Harvey R, Dezi V, Pizzinga M, Willis AE. Harvey R, et al. Biochem Soc Trans. 2017 Aug 15;45(4):1007-14. doi: 10.1042/BST20160364. Epub 2017 Jul 14. Biochem Soc Trans. 2017. PMID: 28710288 Free PMC article. Review.
References
- Niedzwiecka A, Marcotrigiano J, Stepinski J, Jankowska-Anyszka M, Wyslouch-Cieszynska A, Dadlez M, Gingras AC, Mak P, Darzynkiewicz E, Sonenberg N, et al. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol. 2002;319(3):615–635. doi: 10.1016/S0022-2836(02)00328-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous