Wnt pathway regulation of intestinal stem cells - PubMed (original) (raw)
Review
. 2016 Sep 1;594(17):4837-47.
doi: 10.1113/JP271754. Epub 2016 Jun 16.
Affiliations
- PMID: 27581568
- PMCID: PMC5009769
- DOI: 10.1113/JP271754
Review
Wnt pathway regulation of intestinal stem cells
Amanda T Mah et al. J Physiol. 2016.
Abstract
Wnt signalling is involved in multiple aspects of embryonic development and adult tissue homeostasis, notably via controlling cellular proliferation and differentiation. Wnt signalling is subject to stringent positive and negative regulation to promote proper development and homeostasis yet avoid aberrant growth. Such multi-layer regulation includes post-translational modification and processing of Wnt proteins themselves, R-spondin (Rspo) amplification of Wnt signalling, diverse receptor families, and intracellular and extracellular antagonists and destruction and transcription complexes. In the gastrointestinal tract, Wnt signalling is crucial for development and renewal of the intestinal epithelium. Intestinal stem cells (ISCs) undergo symmetric division and neutral drift dynamics to renew the intestinal epithelium. Sources of Wnts and Wnt amplifers such as R-spondins are beginning to be elucidated as well as their functional contribution to intestinal homeostasis. In this review we focus on regulation of ISCs and intestinal homeostasis by the Wnt/Rspo pathway, the potential cellular sources of Wnt signalling regulators and highlight potential future areas of study.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
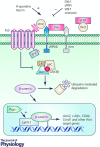
Figure 1. Overview of the canonical Wnt pathway and points of regulation
Wnts bind to their receptors Fzd/Lrp5/6, recruit Dvl and block the destruction complex from degrading cytosolic β‐catenin. Stabilized β‐catenin translocates to the nucleus where it interacts with LEF/TCF to activate Wnt target genes. Wnt antagonists such as Dkk, sFRPs, WIF1 and sclerostin block Wnt signalling by binding and blocking Wnt ligands or their receptors. Norrin and R‐spondins promote Wnt activity by binding or stabilizing membrane availability of Fzd/Lrp5/6. Pygo promotes Wnt signalling by activating transcription via interactions with the complex of proteins bound to the LEF/TCF transcription factors.
Figure 2. The intestinal epithelium contains two functionally distinct pools of intestinal stem cells (ISCs)
Crypt base columnar ISCs (green) and +4 quiescent ISCs (red) co‐exist in the intestinal crypt and differ in their cycling kinetics, sensitivity to extracellular Wnt pathway manipulations and radiation.
Figure 3. R‐spondins potently amplify Wnt signalling
The transmembrane E3 ubiquitin ligases Rnf43/Znrf3 negatively regulate Wnt signalling by ubiquitinating the cytoplasmic tails of Fzds to promote their membrane clearance by endocytosis and degradation, ultimately downregulating Wnt signalling. When R‐spondins (Rspo1–4) are present, Rnf43/Znrf3 mediated degradation of Fzds is inhibited and Fzd/Lrp accumulation on the plasma membrane upregulates Wnt signalling. In an independent mechanism, Rnf43 present on the nuclear membrane interacts with and prevents LEF/TCF‐mediated transcription.
References
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H & Okamoto H (2007). R‐spondin3 is required for mouse placental development. Dev Biol 301, 218–226. - PubMed
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ & Clevers H (2009). Crypt stem cells as the cells‐of‐origin of intestinal cancer. Nature 457, 608–611. - PubMed
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ & Clevers H (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007. - PubMed
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ & Whitsett JA (2008). R‐spondin 2 is required for normal laryngeal‐tracheal, lung and limb morphogenesis. Development 135, 1049–1058. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA151920/CA/NCI NIH HHS/United States
- U01 CA176299/CA/NCI NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- K08 DK096048/DK/NIDDK NIH HHS/United States
- U01 DE025188/DE/NIDCR NIH HHS/United States
- U19 AI116484/AI/NIAID NIH HHS/United States
- U01 DK085527/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical