Reflex control of rat tail sympathetic nerve activity by abdominal temperature - PubMed (original) (raw)
. 2014 Jun 24;1(1):37-41.
doi: 10.4161/temp.29597. eCollection 2014 Apr-Jun.
Affiliations
- PMID: 27583279
- PMCID: PMC4972510
- DOI: 10.4161/temp.29597
Reflex control of rat tail sympathetic nerve activity by abdominal temperature
Anthony D Shafton et al. Temperature (Austin). 2014.
Abstract
The thermoregulatory reflex effects of warming and cooling in the abdomen were investigated in 4 urethane-anesthetized Sprague-Dawley rats. Animals were shaved and surrounded by a water-perfused silastic jacket. Skin temperature under the jacket was recorded by thermocouples at 3 sites and brain temperature was monitored by a thermocouple inserted lateral to the hypothalamus. A heat exchanger made from an array of silicon tubes in parallel loops was placed through a ventral incision into the abdomen; it rested against the intestinal serosa and the temperature of this interface was monitored by a thermocouple. Few- or multi-unit postganglionic activity was recorded from sympathetic nerves supplying tail vessels (tail SNA). Intra-abdominal temperature was briefly lowered or raised between 35-41 °C by perfusing the heat exchanger with cold or warm water. Warming the abdomen inhibited tail SNA while cooling it excited tail SNA in all 4 animals. We also confirmed that cooling the trunk skin activated tail SNA. Multivariate analysis of tail SNA with respect to abdominal, brain and trunk skin temperatures revealed that all had highly significant independent inhibitory actions on tail SNA, but in these experiments abdominal temperature had the weakest and brain temperature the strongest effect. We conclude that abdominal temperature has a significant thermoregulatory action in the rat, but its influence on cutaneous vasomotor control appears to be weaker than that of skin or brain temperatures.
Keywords: Thermoregulation; brain; core; reflex; skin.
Figures
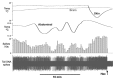
Figure 1. Chart record from one experiment showing brain, trunk skin and abdominal temperatures along with tail SNA as the raw spike record (bottom) and its 10 s spike count (above). The record spans several episodes of actively warming and cooling in the abdomen, which respectively inhibited and excited tail SNA. At the end of the record the trunk skin was cooled via the water jacket, and the resultant tail SNA was silenced by intravenous hexamethonium (Hex), proving that the activity was postganglionic sympathetic.
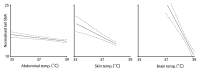
Figure 2. Normalized tail SNA responses to (left to right) abdominal, trunk skin and brain temperatures, calculated by multiple regression analysis of data from the 4 experiments. The respective regression lines are shown with their 95% confidence limits (dashed). All slopes were significantly different from zero (see text).
Similar articles
- Comparison between two rat sympathetic pathways activated in cold defense.
Ootsuka Y, McAllen RM. Ootsuka Y, et al. Am J Physiol Regul Integr Comp Physiol. 2006 Sep;291(3):R589-95. doi: 10.1152/ajpregu.00850.2005. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16601257 - Thermoregulatory control of sympathetic fibres supplying the rat's tail.
Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Owens NC, et al. J Physiol. 2002 Sep 15;543(Pt 3):849-58. doi: 10.1113/jphysiol.2002.023770. J Physiol. 2002. PMID: 12231643 Free PMC article. - Independent vasomotor control of rat tail and proximal hairy skin.
Tanaka M, Ootsuka Y, McKinley MJ, McAllen RM. Tanaka M, et al. J Physiol. 2007 Jul 1;582(Pt 1):421-33. doi: 10.1113/jphysiol.2007.131292. Epub 2007 Apr 12. J Physiol. 2007. PMID: 17430987 Free PMC article. - Cold-activated raphé-spinal neurons in rats.
Rathner JA, Owens NC, McAllen RM. Rathner JA, et al. J Physiol. 2001 Sep 15;535(Pt 3):841-54. doi: 10.1111/j.1469-7793.2001.t01-1-00841.x. J Physiol. 2001. PMID: 11559779 Free PMC article. - Effect of capsaicin on thermoregulation: an update with new aspects.
Szolcsányi J. Szolcsányi J. Temperature (Austin). 2015 Jun 2;2(2):277-96. doi: 10.1080/23328940.2015.1048928. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227029 Free PMC article. Review.
Cited by
- Central nervous system circuits that control body temperature.
Madden CJ, Morrison SF. Madden CJ, et al. Neurosci Lett. 2019 Mar 23;696:225-232. doi: 10.1016/j.neulet.2018.11.027. Epub 2018 Dec 23. Neurosci Lett. 2019. PMID: 30586638 Free PMC article. Review. - Hypothalamic temperature of rats subjected to treadmill running in a cold environment.
Fonseca CG, Pires W, Lima MR, Guimarães JB, Lima NR, Wanner SP. Fonseca CG, et al. PLoS One. 2014 Nov 3;9(11):e111501. doi: 10.1371/journal.pone.0111501. eCollection 2014. PLoS One. 2014. PMID: 25365556 Free PMC article. - Thermal physiology in a changing thermal world.
Horowitz M, Kenny GP, McAllen RM, van Marken Lichtenbelt WD. Horowitz M, et al. Temperature (Austin). 2015 Apr 14;2(1):22-6. doi: 10.1080/23328940.2015.1017088. eCollection 2015 Jan-Mar. Temperature (Austin). 2015. PMID: 27226998 Free PMC article. - Regulation of Body Temperature by the Nervous System.
Tan CL, Knight ZA. Tan CL, et al. Neuron. 2018 Apr 4;98(1):31-48. doi: 10.1016/j.neuron.2018.02.022. Neuron. 2018. PMID: 29621489 Free PMC article. Review.
References
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000 Dec 20;85(1-3):18-25 - PubMed
- Romanovsky AA. . Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 2007; 292:R37 - 46; http://dx.doi.org/10.1152/ajpregu.00668.2006; PMID: 17008453 - DOI - PubMed
- Jessen C. . Thermal afferents in the control of body temperature. Pharmacol Ther 1985; 28:107 - 34; http://dx.doi.org/10.1016/0163-7258(85)90085-3; PMID: 4059328 - DOI - PubMed
- Jessen C, Simon E. Spinal cord and hypothalamus as core sensors of temperature in the conscious dog. 3. Identity of functions. Pflugers Arch. 1971;324(3):217-26 - PubMed
- McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. . Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol 2010; 109:27 - 33; http://dx.doi.org/10.1007/s00421-009-1295-z; PMID: 19949811 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials