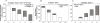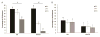Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering - PubMed (original) (raw)
Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering
Nelda Vázquez-Portalatı N et al. Biomacromolecules. 2016.
Abstract
Biomaterials that provide signals present in the native extracellular matrix have been proposed as scaffolds to support improved cartilage regeneration. This study harnesses the biological activity of collagen type II and the superior mechanical properties of collagen type I by characterizing gels made of collagen type I and II blends. The collagen blend hydrogels were able to incorporate both types of collagen and retained chondroitin sulfate and hyaluronic acid. Cryo-scanning electron microscopy images showed that the 3:1 ratio of collagen type I to type II gels had a lower void space percentage (36.4%) than the 1:1 gels (46.5%). The complex modulus was larger for the 3:1 gels (G* = 5.0 Pa) compared to the 1:1 gels (G* = 1.2 Pa). The 3:1 blend consistently formed gels with superior mechanical properties compared to the other blends and has the potential to be implemented as a scaffold for articular cartilage engineering.
Figures
Figure 1
(A) The final collagen concentration in the gel at different ratio blends uses white bars to represent collagen type I found in fibrillary form and gray bars to represent collagen type II measured in fibrillary form. Data (n = 3) are represented as the mean ± the standard deviation of the total concentration of collagen (both collagen types I and II) in the gel. An ANOVA and Tukey’s honestly significant difference_post hoc_ test were performed and indicate a significant difference in the total protein concentration in the gel between each ratio (p < 0.05). The final concentration of collagen in the gel and supernatant for (B) collagen type I and (C) collagen type II. The white bars represent collagen content in fibrillar form, whereas the gray bars represent collagen measured in the supernatant (n = 3). The error bars represent the standard deviation of the amount of collagen type II in the supernatant.
Figure 2
The final protein concentration in the fibrils at different ratio blends of collagen type I and collagen type II with the addition of HA, CS, or both HA and CS. The gels were created containing a (A) 3:1, (B) 1:1, or (C) 1:3 ratio of collagen type I to collagen type II. An ANOVA and Tukey’s honestly significant difference post hoc tests were performed. Different letters indicate groups with significantly different total protein concentrations incorporated in the gel (p < 0.05). Data (n = 3) are represented as the mean ± the standard deviation.
Figure 3
The percentage of (A) CS and (B) HA retained in the fibrils. Gels were created using varying ratios of collagen type I to II (3:1, 1:1, and 1:3) with either CS, HA, or both CS and HA added into the gels. ANOVA and Tukey’s honestly significant difference_post hoc_ tests were performed. The * indicates significant differences (p < 0.05) between the three different hydrogel blends when CS is added. The # indicates significant differences (p < 0.05) between the three different hydrogel blends when HA and CS are added. EE indicates there is no statistical difference (p > 0.05) between the 3:1 gels. Ff and Gg indicate that there were statistical differences (p < 0.05) between the 1:1 and 1:3 gels, respectively. There is no significant difference (p > 0.05) between the three different hydrogel blends when either HA or HA and CS were added. Hh and Jj indicate that there were statistical differences (p < 0.05) between the 3:1 and 1:3 gels, respectively. II indicates there is no statistical difference (p > 0.05) between the 1:1 gels. Data (n = 3) are represented as the mean ± the standard deviation.
Figure 4
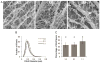
Collagen networks for different ratio blends. (A) Representative cryoSEM images of different ratio blends of collagen type I to collagen type II show the collagen fibril network within the gels. Scale bar represents 5μm. (B) Distribution of collagen fibril diameters in the gels at different ratio blends. (C) Percentage of void space for the gels based on cryoSEM images obtained at 10,000x magnification. ANOVA and Tukey’s post hoc tests were performed on the percentage of void space data by using nested factorial models. The different letters indicate groups with a significant difference (p < 0.05) in the percentage of void space in the gels. Data (n ≥ 15) for void space percentage are represented as the mean ± the standard deviation.
Figure 5
Frequency sweeps of storage and loss moduli of gels prepared from mixtures of (A) 1:0, (B) 3:1, and (C) 1:1 collagen type I to collagen type II. ANOVA and Tukey’s_post hoc_ tests were performed. At 1 Hz, a significant difference (p < 0.05) was observed between the storage moduli for the three different gel blends. At 1 Hz, the loss modulus for the 1:1 gels was significantly different (p < 0.05) from the loss moduli of the 1:0 and 3:1 gels. Data (n = 4) are represented as the mean ± the standard deviation.
Similar articles
- Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage.
Nguyen LH, Kudva AK, Guckert NL, Linse KD, Roy K. Nguyen LH, et al. Biomaterials. 2011 Feb;32(5):1327-38. doi: 10.1016/j.biomaterials.2010.10.009. Epub 2010 Nov 10. Biomaterials. 2011. PMID: 21067807 - Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures.
Amann E, Wolff P, Breel E, van Griensven M, Balmayor ER. Amann E, et al. Acta Biomater. 2017 Apr 1;52:130-144. doi: 10.1016/j.actbio.2017.01.064. Epub 2017 Jan 25. Acta Biomater. 2017. PMID: 28131943 - Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering.
Guo Y, Yuan T, Xiao Z, Tang P, Xiao Y, Fan Y, Zhang X. Guo Y, et al. J Mater Sci Mater Med. 2012 Sep;23(9):2267-79. doi: 10.1007/s10856-012-4684-5. Epub 2012 May 26. J Mater Sci Mater Med. 2012. PMID: 22639153 - Should we use cells, biomaterials, or tissue engineering for cartilage regeneration?
Bernhard JC, Vunjak-Novakovic G. Bernhard JC, et al. Stem Cell Res Ther. 2016 Apr 18;7(1):56. doi: 10.1186/s13287-016-0314-3. Stem Cell Res Ther. 2016. PMID: 27089917 Free PMC article. Review. - Nanomaterials and hydrogel scaffolds for articular cartilage regeneration.
Reddi AH, Becerra J, Andrades JA. Reddi AH, et al. Tissue Eng Part B Rev. 2011 Oct;17(5):301-5. doi: 10.1089/ten.TEB.2011.0141. Epub 2011 Aug 29. Tissue Eng Part B Rev. 2011. PMID: 21595612 Review.
Cited by
- Intensified Stiffness and Photodynamic Provocation in a Collagen-Based Composite Hydrogel Drive Chondrogenesis.
Zheng L, Liu S, Cheng X, Qin Z, Lu Z, Zhang K, Zhao J. Zheng L, et al. Adv Sci (Weinh). 2019 Jul 15;6(16):1900099. doi: 10.1002/advs.201900099. eCollection 2019 Aug 21. Adv Sci (Weinh). 2019. PMID: 31453055 Free PMC article. - Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research.
Baumgartner L, Wuertz-Kozak K, Le Maitre CL, Wignall F, Richardson SM, Hoyland J, Ruiz Wills C, González Ballester MA, Neidlin M, Alexopoulos LG, Noailly J. Baumgartner L, et al. Int J Mol Sci. 2021 Jan 12;22(2):703. doi: 10.3390/ijms22020703. Int J Mol Sci. 2021. PMID: 33445782 Free PMC article. Review. - Incorporation of a Collagen-Binding Chondroitin Sulfate Molecule to a Collagen Type I and II Blend Hydrogel for Cartilage Tissue Engineering.
Kilmer CE, Walimbe T, Panitch A, Liu JC. Kilmer CE, et al. ACS Biomater Sci Eng. 2022 Mar 14;8(3):1247-1257. doi: 10.1021/acsbiomaterials.1c01248. Epub 2022 Feb 8. ACS Biomater Sci Eng. 2022. PMID: 35133126 Free PMC article. - The enhancement of cartilage regeneration by use of a chitosan-based scaffold in a 3D model of microfracture in vitro: a pilot evaluation.
Andjelkov N, Riyadh H, Ivarsson M, Kacarevic-Popovic Z, Krstic J, Wretenberg P. Andjelkov N, et al. J Exp Orthop. 2021 Feb 18;8(1):12. doi: 10.1186/s40634-021-00328-z. J Exp Orthop. 2021. PMID: 33599885 Free PMC article. No abstract available. - Extraction and Characterization of Collagen from Elasmobranch Byproducts for Potential Biomaterial Use.
Seixas MJ, Martins E, Reis RL, Silva TH. Seixas MJ, et al. Mar Drugs. 2020 Dec 4;18(12):617. doi: 10.3390/md18120617. Mar Drugs. 2020. PMID: 33291538 Free PMC article.
References
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Deyo RA, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. - PMC - PubMed
- Wang Z, Peng JJ. Articular cartilage tisse engineering: development and future: a review. Musculoskelet Pain. 2014;22:68–77.
- Hutmacher DW. Scaffold in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. - PubMed
- Rahman RA, Radzi MAA, Sukri NM, Nazir NM, Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng Regen Med. 2015;12:1–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources