The NIH Toolbox Cognitive Battery for intellectual disabilities: three preliminary studies and future directions - PubMed (original) (raw)
The NIH Toolbox Cognitive Battery for intellectual disabilities: three preliminary studies and future directions
David Hessl et al. J Neurodev Disord. 2016.
Abstract
Background: Recent advances in understanding molecular and synaptic mechanisms of intellectual disabilities (ID) in fragile X syndrome (FXS) and Down syndrome (DS) through animal models have led to targeted controlled trials with pharmacological agents designed to normalize these underlying mechanisms and improve clinical outcomes. However, several human clinical trials have failed to demonstrate efficacy of these targeted treatments to improve surrogate behavioral endpoints. Because the ultimate index of disease modification in these disorders is amelioration of ID, the validation of cognitive measures for tracking treatment response is essential. Here, we present preliminary research to validate the National Institutes of Health Toolbox Cognitive Battery (NIH-TCB) for ID.
Methods: We completed three pilot studies of patients with FXS (total n = 63; mean age 19.3 ± 8.3 years, mean mental age 5.3 ± 1.6 years), DS (n = 47; mean age 16.1 ± 6.2, mean mental age 5.4 ± 2.0), and idiopathic ID (IID; n = 16; mean age 16.1 ± 5.0, mean mental age 6.6 ± 2.3) measuring processing speed, executive function, episodic memory, word/letter reading, receptive vocabulary, and working memory using the web-based NIH-TB-CB, addressing feasibility, test-retest reliability, construct validity, ecological validity, and syndrome differences and profiles.
Results: Feasibility was good to excellent (≥80 % of participants with valid scores) for above mental age 4 years for all tests except list sorting (working memory). Test-retest stability was good to excellent, and convergent validity was similar to or better than results obtained from typically developing children in the normal sample for executive function and language measures. Examination of ecological validity revealed moderate to very strong correlations between the NIH-TCB composite and adaptive behavior and full-scale IQ measures. Syndrome/group comparisons demonstrated significant deficits for the FXS and DS groups relative to IID on attention and inhibitory control, a significant reading weakness for FXS, and a receptive vocabulary weakness for DS.
Conclusions: The NIH-TCB has potential for assessing important dimensions of cognition in persons with ID, and several tests may be useful for tracking response to intervention. However, more extensive psychometric studies, evaluation of the NIH-TCB's sensitivity to change, both developmentally and in the context of treatment, and perhaps establishing links to brain function in these populations, are required to determine the true utility of the battery as a set of outcome measures.
Keywords: Assessment; Cognition; Down syndrome; FMR1 gene; Fragile X syndrome; Outcome measures.
Figures
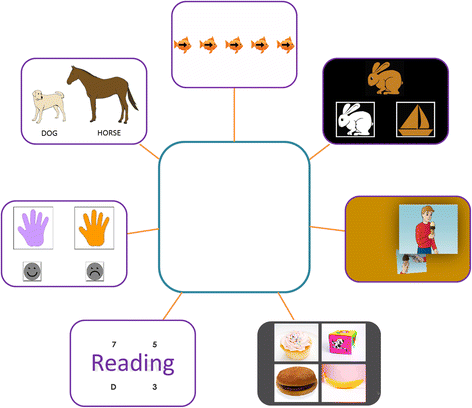
Fig. 1
Story board/picture schedule used in study 3 to increase motivation and understanding of assessment visit schedule. The participant prize or a representation of the prize for compliance and effort is placed in the center, and with the examiner’s assistance, the participant checks off each completed task with a dry erase pen. NIH-TCB tasks from top (clockwise) depicted are flanker, dimensional change card sort, picture sequence memory, picture vocabulary, oral reading, pattern comparison, and list sorting
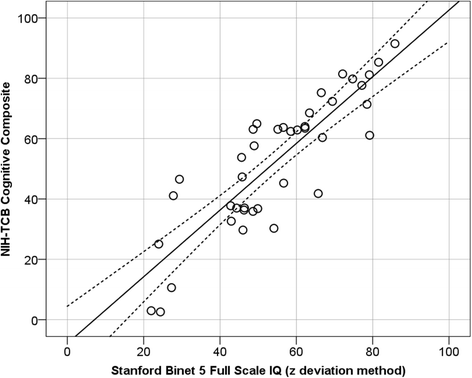
Fig. 2
Scatterplot showing the association between the NIH-TCB cognitive composite and Stanford-Binet full-scale IQ (z deviation method). Dotted lines represent the 95 % confidence interval around the regression line. Note that the regression line and correlation (−12.76 + 1.17×; R 2 = .79) show that the composite is a strong predictor of IQ in these samples of individuals with ID
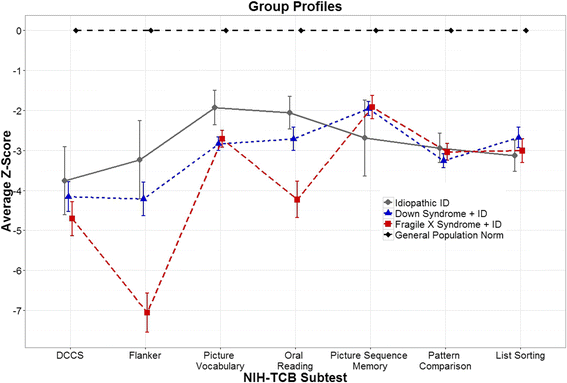
Fig. 3
Z scores (±1 SEM) of each NIH-TCB subtest by group. Z scores (age-adjusted) reflect the number of standard deviations from the average (0 for all subtests) in the normative sample from the general population. For example, the FXS + ID sample had a mean performance on flanker that is greater than 7 standard deviations below average, adjusted for age. Note that for picture sequence memory, only data from form A is shown
Similar articles
- Validation of the NIH Toolbox Cognitive Battery in intellectual disability.
Shields RH, Kaat AJ, McKenzie FJ, Drayton A, Sansone SM, Coleman J, Michalak C, Riley K, Berry-Kravis E, Gershon RC, Widaman KF, Hessl D. Shields RH, et al. Neurology. 2020 Mar 24;94(12):e1229-e1240. doi: 10.1212/WNL.0000000000009131. Epub 2020 Feb 24. Neurology. 2020. PMID: 32094241 Free PMC article. - Sensitivity of the NIH Toolbox to Detect Cognitive Change in Individuals With Intellectual and Developmental Disability.
Shields RH, Kaat A, Sansone SM, Michalak C, Coleman J, Thompson T, McKenzie FJ, Dakopolos A, Riley K, Berry-Kravis E, Widaman KF, Gershon RC, Hessl D. Shields RH, et al. Neurology. 2023 Feb 21;100(8):e778-e789. doi: 10.1212/WNL.0000000000201528. Epub 2022 Dec 2. Neurology. 2023. PMID: 36460468 Free PMC article. - Validity of the NIH toolbox cognitive battery in a healthy oldest-old 85+ sample.
Nolin SA, Cowart H, Merritt S, McInerney K, Bharadwaj PK, Franchetti MK, Raichlen DA, Jessup CJ, Hishaw GA, Van Etten EJ, Trouard TP, Geldmacher DS, Wadley VG, Porges ES, Woods AJ, Cohen RA, Levin BE, Rundek T, Alexander GE, Visscher KM. Nolin SA, et al. J Int Neuropsychol Soc. 2023 Jul;29(6):605-614. doi: 10.1017/S1355617722000443. Epub 2022 Oct 14. J Int Neuropsychol Soc. 2023. PMID: 36239453 Free PMC article. - Updated report on tools to measure outcomes of clinical trials in fragile X syndrome.
Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, King MK, Abbeduto L, Kaufmann WE. Budimirovic DB, et al. J Neurodev Disord. 2017 Jun 12;9:14. doi: 10.1186/s11689-017-9193-x. eCollection 2017. J Neurodev Disord. 2017. PMID: 28616097 Free PMC article. Review. - Intellectual functioning and behavior in Dravet syndrome: A systematic review.
Jansson JS, Hallböök T, Reilly C. Jansson JS, et al. Epilepsy Behav. 2020 Jul;108:107079. doi: 10.1016/j.yebeh.2020.107079. Epub 2020 Apr 22. Epilepsy Behav. 2020. PMID: 32334365
Cited by
- Inhibition of phosphodiesterase-4D in adults with fragile X syndrome: a randomized, placebo-controlled, phase 2 clinical trial.
Berry-Kravis EM, Harnett MD, Reines SA, Reese MA, Ethridge LE, Outterson AH, Michalak C, Furman J, Gurney ME. Berry-Kravis EM, et al. Nat Med. 2021 May;27(5):862-870. doi: 10.1038/s41591-021-01321-w. Epub 2021 Apr 29. Nat Med. 2021. PMID: 33927413 Clinical Trial. - Evaluating Verbal Fluency Outcome Measures in Children With Down Syndrome.
Smeyne CN, Esbensen AJ, Schworer EK, Belizaire S, Hoffman EK, Beebe DW, Wiley S. Smeyne CN, et al. Am J Intellect Dev Disabil. 2022 Jul 1;127(4):328-344. doi: 10.1352/1944-7558-127.4.328. Am J Intellect Dev Disabil. 2022. PMID: 36122330 Free PMC article. - Deviation scores: An innovative approach to interpreting cognitive test results for individuals with intellectual disabilities.
Talapatra D, Snider L, Coleman J, Thompson T, Reinhardt JS, Hessl D, Riley K. Talapatra D, et al. J Appl Res Intellect Disabil. 2023 Nov;36(6):1218-1228. doi: 10.1111/jar.13137. Epub 2023 Aug 8. J Appl Res Intellect Disabil. 2023. PMID: 37553958 Free PMC article. Review. - Parental Education, Household Income, Race, and Children's Working Memory: Complexity of the Effects.
Akhlaghipour G, Assari S. Akhlaghipour G, et al. Brain Sci. 2020 Dec 7;10(12):950. doi: 10.3390/brainsci10120950. Brain Sci. 2020. PMID: 33297546 Free PMC article. - Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy.
Shalabi H, Wolters PL, Martin S, Toledo-Tamula MA, Roderick MC, Struemph K, Kane E, Yates B, Delbrook C, Mackall CL, Lee DW, Fry TJ, Shah NN. Shalabi H, et al. J Immunother. 2018 Sep;41(7):350-358. doi: 10.1097/CJI.0000000000000241. J Immunother. 2018. PMID: 30048343 Free PMC article. Clinical Trial.
References
- Goldberg EM. Are the majority of children with autism mentally retarded: a systematic evaluation of the data. Focus Autism Dev Dis. 2011;21(2):66–83. doi: 10.1177/10883576060210020301. - DOI
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10(4):411–413. - PubMed
Grants and funding
- TL1 TR000133/TR/NCATS NIH HHS/United States
- U54 HD079125/HD/NICHD NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- R01 HD076189/HD/NICHD NIH HHS/United States
- UL1 TR000153/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- UL1 RR024146/RR/NCRR NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources