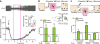Locus coeruleus and dopaminergic consolidation of everyday memory - PubMed (original) (raw)
. 2016 Sep 15;537(7620):357-362.
doi: 10.1038/nature19325. Epub 2016 Sep 7.
Affiliations
- PMID: 27602521
- PMCID: PMC5161591
- DOI: 10.1038/nature19325
Locus coeruleus and dopaminergic consolidation of everyday memory
Tomonori Takeuchi et al. Nature. 2016.
Abstract
The retention of episodic-like memory is enhanced, in humans and animals, when something novel happens shortly before or after encoding. Using an everyday memory task in mice, we sought the neurons mediating this dopamine-dependent novelty effect, previously thought to originate exclusively from the tyrosine-hydroxylase-expressing (TH+) neurons in the ventral tegmental area. Here we report that neuronal firing in the locus coeruleus is especially sensitive to environmental novelty, locus coeruleus TH+ neurons project more profusely than ventral tegmental area TH+ neurons to the hippocampus, optogenetic activation of locus coeruleus TH+ neurons mimics the novelty effect, and this novelty-associated memory enhancement is unaffected by ventral tegmental area inactivation. Surprisingly, two effects of locus coeruleus TH+ photoactivation are sensitive to hippocampal D1/D5 receptor blockade and resistant to adrenoceptor blockade: memory enhancement and long-lasting potentiation of synaptic transmission in CA1 ex vivo. Thus, locus coeruleus TH+ neurons can mediate post-encoding memory enhancement in a manner consistent with possible co-release of dopamine in the hippocampus.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Figures
Figure 1. Novelty exploration after memory encoding enhances memory retention
a, Everyday spatial memory task in event arena. Mice [_n_=13; 100 sessions (Ss)) learned and maintained stable performance (S25–S100: _F_14,168=1.68, _P_>0.05). Non-encoding control session performance(S63, orange arrow) dropped to 59.6% (S61–S65: _F_4,48=3.63, P<0.05; S63: _t_-test vs. chance, _t_12<1). Green circles, 5S average; white circles, 1S average; Pre, pre-training. **b**, Memory persistence (probe tests) declined from memory at 1h to chance at 24h (1h vs. 24h: _t_12=2.94, _P_<0.05; 1h: _t_12=4.44, _P_<0.001; 24h: _t_12=1.42, _P_>0.05). Novelty 30 min after encoding resulted in memory at 24h (No novelty vs. Novelty: _t_12=2.24, P<0.05; Novelty: _t_12=3.17, P<0.01). c, Blockade of hippocampal D1/D5 receptor (SCH) but not β-adrenoceptors (Prop) during novelty abolished 24h memory (_F_2,24=3.83, P<0.05; Vehicle: _t_12=4.38, P<0.001; Prop: _t_12=3.33, P<0.01). HPC, hippocampus; Prop, propranolol; SCH, SCH23390. **P<0.01, ***P<0.001 vs. chance. Dashed lines=chance level. Means±s.e.m.
Figure 2. LC-TH+ neurons show stronger modulation by novelty than VTA-TH+ neurons
a, Viral injection and optetrode implantation. Putative VTA-TH+ and LC-TH+ neurons responded to blue light (blue). b, Behavioural protocol. c, Raster plot of VTA-TH+ (top) and LC-TH+ neurons (bottom) in familiar (left) and novel (right) environments. FR, mean firing rate. d, Firing rates of VTA-TH+ (_n_=15 neurons from 5 mice) and LC-TH+ (_n_=10 neurons from 3 mice) neurons were higher in the novel environment (VTA-TH+: _t_14=4.31, P<0.01; LC-TH+: _t_9=3.45, _P_<0.01). Dashed lines=baseline. **e**, LC-TH+ neurons showed stronger modulation by novelty than VTA-TH+ neurons (Brain Area×Condition interaction, _F_29,667=2.28, _P_<0.001). LC-TH+ but not VTA-TH+ neurons displayed habituation to novelty (Brain Area×Condition×Time interaction, _F_29,667=2.03, _P_<0.01; main effect of Time for LC novel, _F_29,261=1.70, _P_<0.05; for VTA novel, _F_29,406=1.24, _P_>0.05). **P<0.01, paired _t_-test. Means±s.e.m.
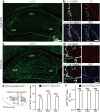
Figure 3. TH+ axons in the hippocampus originate from LC-TH+ neurons
a–d, Representative coronal sections show overall distribution of eYFP+ axons from VTA (a) and LC (c) in dorsal hippocampus. Triple immunofluorescence for eYFP (green), TH (red) and NET (blue) shows co-labelling of eYFP+ VTA axons with TH (b, top) but not with NET (b, bottom; arrows), co-labelling of eYFP+ LC axons with TH (d, top) and NET (d, bottom). e, f, Quantification of area occupied by eYFP and TH double-positive axons (e), and the ratio of eYFP and TH double-positive axons relative to all TH+ axons (f) in CA1, CA3, and DG (_n_=9 slices from 3 mice/group). Both measures indicate stronger TH+ projections from LC than from VTA in CA1 (Area: _t_16=7.4, P<0.001; Ratio: _t_16=104.1, P<0.001), CA3 (Area: _t_16=11.7, P<0.001; Ratio: _t_16=59.0, P<0.001) and DG (Area: _t_16=10.8, P<0.001; Ratio: _t_16=76.4, P<0.001). ***P<0.001, paired _t_-test. Means±s.e.m.
Figure 4. Optogenetic activation of LC-TH+ neurons enhances memory persistence
a, Viral injection, and optic fibre and drug cannulae implantations. Th-Cre mice were injected with a Cre-inducible ChR2-eYFP AAV (ChR2+, _n_=8) or a control eYFP AAV (ChR2−, _n_=6) into LC and VTA. b, Optogenetic burst protocol used in the event arena experiment. c, Design for the optogenetic mimicry experiment. d, Left, LC-TH+ neuron photostimulation (LC-ON) 30 min after encoding enhanced 24-h memory in ChR2+ animals but not in ChR2− controls (Group×Condition interaction, _F_1,12=5.66, P<0.05; ChR2+ in LC-ON vs. chance: _t_7=4.38, P<0.01). Right, VTA-TH+ neuron photostimulation (VTA-ON) caused a trend for enhanced memory that did not differ between groups (Group×Condition interaction, _F_1,12=0.33, _P_=0.58; ChR2+ in VTA-ON: _t_7=2.22, _P_=0.062; ChR2− in VTA-ON: _t_5=1.55, _P_=0.18). e, Design for the optogenetic LC activation experiment with pharmacological interventions. f, Blockade of hippocampal D1/D5 receptor (SCH) but not β-adrenoceptors (Prop) during LC-TH+ neuron photostimulation abolished the effect of LC photostimulation on memory persistence in ChR2+ mice (Group effect, _F_1,12=5.01, P<0.05; Condition effect in ChR2+, _F_3,21=3.18, P<0.05; in ChR2−: _F_3,15<1). ChR2+ mice showed good memory with post-encoding LC-ON in presence of vehicle or Prop, but not in presence of SCH or without light stimulation (Orthogonal comparisons, _F_3,21=9.23, P<0.01; LC-ON with Vehicle in ChR2+: _t_7=3.01, P<0.05; LC-ON with Prop in ChR2+: _t_7=2.41, P<0.05). Prop, propranolol; SCH, SCH23390. *P<0.05, **P<0.01 vs. chance. Dashed lines=chance level. Means±s.e.m.
Figure 5. Optogenetic activation of LC-TH+ axons enhances hippocampal synaptic function
a, Hippocampal slice physiology. Orange line=hippocampal slice plane. b, Left, potentiation of Schaffer collateral (SC)-evoked EPSCs from CA1 pyramidal neurons after strong optogenetic activation (blue) of hippocampal LC-TH+ axons (Light-ON, _n_=5) is unaffected by adrenoceptor antagonists (Light-ON with Praz/Prop, _n_=4) but blocked by D1/D5 receptor antagonist (Light-ON with SCH, _n_=5) (Conditions×Time interaction, _F_7.8, 42.8=2.50, P<0.05, Greenhouse-Geisser correction). Middle, exemplar EPSCs from CA1 pyramidal neurons. Dashed lines=baseline EPSCs; Continuous=EPSCs 30–35 min after the optogenetic stimulation onset. Right, mean EPSCs 35 min after the optogenetic stimulation onset, showing effect of D1/D5 receptor antagonist (SCH) but no effect of adrenoceptor antagonists (Praz/Prop) (_F_2,11=6.38, _P_<0.05). **c**, Left, fEPSP responses to weak theta-burst stimulation (arrow) with or without optogenetic activation of hippocampal LC-TH+ axons (blue). No synaptic potentiation without theta-burst [Light-ON (no LTP), _n_=6], but with it, an increase in synaptic strength lasting > 45 min (Light-OFF + LTP, _n_=6) that was significantly enhanced by a weak physiologically relevant optogenetic stimulation of LC-TH+ axons (Light-ON + LTP, _n_=11). SCH blocked optogenetic enhancement of LTP (Light-ON + LTP with SCH, _n_=5) (Conditions×Time interaction, F19.6/156.7=2.93, P<0.001). Middle, fEPSPs: baseline (dashed lines) and 40–45 min after theta-burst stimulation (continuous lines). Right, mean fEPSP slopes 45 min after theta-burst stimulation, shows blockade of optogenetic augmentation of LTP by SCH (_F_3,24=16.99, P<0.001). Praz, prazosin; Prop, propranolol; SCH, SCH23390 or SCH39166 (see Methods). ns, not significant. *P<0.05, ***P<0.001, Tukey HSD test. Means±s.e.m.
Figure 6. Pharmacological inhibition of VTA has no impact on the novelty effect
a, Microinfusion of lidocaine (Lid) into VTA blocks multi-unit activity (example trace and population data; _n_=8 traces/4 mice) (Pre vs. Lid: _t_7=8.42, P<0.001; Pre vs. Post: _t_7=1.42, _P_>0.05). Grey shading represents±s.e.m. Magenta box, novelty period. b, Lidocaine into the VTA before novelty had no effect on memory enhancement (_n_=15 mice) (Vehicle vs. Lid: _t_14<1, _P_>0.05; Vehicle vs. chance: _t_14=2.95, P<0.05; Lid: _t_14=2.19, P<0.05). c, Systemic injection of α2-adrenergic receptor agonist clonidine before novelty abolishes the novelty effect (_F_2,28=7.70, P<0.01; Novelty with Vehicle: _t_14=4.62, P<0.001). ns, not significant. *P<0.05, ***P<0.001. Dashed lines=chance level. Means±s.e.m.
Comment in
- Two sources of dopamine for the hippocampus.
McNamara CG, Dupret D. McNamara CG, et al. Trends Neurosci. 2017 Jul;40(7):383-384. doi: 10.1016/j.tins.2017.05.005. Epub 2017 May 13. Trends Neurosci. 2017. PMID: 28511793 Free PMC article.
Similar articles
- Locus Coeruleus and Dopamine-Dependent Memory Consolidation.
Yamasaki M, Takeuchi T. Yamasaki M, et al. Neural Plast. 2017;2017:8602690. doi: 10.1155/2017/8602690. Epub 2017 Oct 16. Neural Plast. 2017. PMID: 29123927 Free PMC article. Review. - Cell-type-specific optogenetic stimulation of the locus coeruleus induces slow-onset potentiation and enhances everyday memory in rats.
Tse D, Privitera L, Norton AC, Gobbo F, Spooner P, Takeuchi T, Martin SJ, Morris RGM. Tse D, et al. Proc Natl Acad Sci U S A. 2023 Nov 14;120(46):e2307275120. doi: 10.1073/pnas.2307275120. Epub 2023 Nov 6. Proc Natl Acad Sci U S A. 2023. PMID: 37931094 Free PMC article. - Novelty and Dopaminergic Modulation of Memory Persistence: A Tale of Two Systems.
Duszkiewicz AJ, McNamara CG, Takeuchi T, Genzel L. Duszkiewicz AJ, et al. Trends Neurosci. 2019 Feb;42(2):102-114. doi: 10.1016/j.tins.2018.10.002. Epub 2018 Nov 16. Trends Neurosci. 2019. PMID: 30455050 Free PMC article. Review. - Oppositional and competitive instigation of hippocampal synaptic plasticity by the VTA and locus coeruleus.
Hagena H, Manahan-Vaughan D. Hagena H, et al. Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2402356122. doi: 10.1073/pnas.2402356122. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793037 Free PMC article. - Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory.
Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Kempadoo KA, et al. Proc Natl Acad Sci U S A. 2016 Dec 20;113(51):14835-14840. doi: 10.1073/pnas.1616515114. Epub 2016 Dec 7. Proc Natl Acad Sci U S A. 2016. PMID: 27930324 Free PMC article.
Cited by
- Contextual memory engrams, and the neuromodulatory influence of the locus coeruleus.
Grella SL, Donaldson TN. Grella SL, et al. Front Mol Neurosci. 2024 Feb 5;17:1342622. doi: 10.3389/fnmol.2024.1342622. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38375501 Free PMC article. Review. - A computational model to explore how temporal stimulation patterns affect synapse plasticity.
Amano R, Nakao M, Matsumiya K, Miwakeichi F. Amano R, et al. PLoS One. 2022 Sep 23;17(9):e0275059. doi: 10.1371/journal.pone.0275059. eCollection 2022. PLoS One. 2022. PMID: 36149886 Free PMC article. - Dopamine is associated with prioritization of reward-associated memories in Parkinson's disease.
Sharp ME, Duncan K, Foerde K, Shohamy D. Sharp ME, et al. Brain. 2020 Aug 1;143(8):2519-2531. doi: 10.1093/brain/awaa182. Brain. 2020. PMID: 32844197 Free PMC article. - Defining circuit-specific roles for G protein-coupled receptors in aversive learning.
Gowrishankar R, Bruchas MR. Gowrishankar R, et al. Curr Opin Behav Sci. 2019 Apr;26:146-156. doi: 10.1016/j.cobeha.2019.01.002. Epub 2019 Feb 8. Curr Opin Behav Sci. 2019. PMID: 32855999 Free PMC article. - Reduction in the activity of VTA/SNc dopaminergic neurons underlies aging-related decline in novelty seeking.
Shan Q, Tian Y, Chen H, Lin X, Tian Y. Shan Q, et al. Commun Biol. 2023 Dec 2;6(1):1224. doi: 10.1038/s42003-023-05571-x. Commun Biol. 2023. PMID: 38042964 Free PMC article.
References
- Wixted JT. The psychology and neuroscience of forgetting. Annu Rev Psychol. 2004;55:235–269. - PubMed
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. - PubMed
- Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. - PubMed
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12024/3/MRC_/Medical Research Council/United Kingdom
- T32 DA007290/DA/NIDA NIH HHS/United States
- 268800/ERC_/European Research Council/International
- MC_UU_12020/7/MRC_/Medical Research Council/United Kingdom
- R01 MH080297/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous