Ketamine Treatment and Global Brain Connectivity in Major Depression - PubMed (original) (raw)
. 2017 May;42(6):1210-1219.
doi: 10.1038/npp.2016.186. Epub 2016 Sep 8.
Lynnette A Averill 1 2, Katherine A Collins 3, Paul Geha 1 2, Jaclyn Schwartz 4, Christopher Averill 1 2, Kaitlin E DeWilde 4, Edmund Wong 5, Alan Anticevic 1 2 6 7, Cheuk Y Tang 5, Dan V Iosifescu 3 4 8, Dennis S Charney 3 4 8, James W Murrough 3 4 8
Affiliations
- PMID: 27604566
- PMCID: PMC5437875
- DOI: 10.1038/npp.2016.186
Ketamine Treatment and Global Brain Connectivity in Major Depression
Chadi G Abdallah et al. Neuropsychopharmacology. 2017 May.
Abstract
Capitalizing on recent advances in resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) and the distinctive paradigm of rapid mood normalization following ketamine treatment, the current study investigated intrinsic brain networks in major depressive disorder (MDD) during a depressive episode and following treatment with ketamine. Medication-free patients with MDD and healthy control subjects (HC) completed baseline rs-fcMRI. MDD patients received a single infusion of ketamine and underwent repeated rs-fcMRI at 24 h posttreatment. Global brain connectivity with global signal regression (GBCr) values were computed as the average of correlations of each voxel with all other gray matter voxels in the brain. MDD group showed reduced GBCr in the prefrontal cortex (PFC) but increased GBCr in the posterior cingulate, precuneus, lingual gyrus, and cerebellum. Ketamine significantly increased GBCr in the PFC and reduced GBCr in the cerebellum. At baseline, 2174 voxels of altered GBCr were identified, but only 310 voxels significantly differed relative to controls following treatment (corrected α<0.05). Responders to ketamine showed increased GBCr in the lateral PFC, caudate, and insula. Follow-up seed-based analyses illustrated a pattern of dysconnectivity between the PFC/subcortex and the rest of the brain in MDD, which appeared to normalize postketamine. The extent of the functional dysconnectivity identified in MDD and the swift and robust normalization following treatment suggest that GBCr may serve as a treatment response biomarker for the development of rapid acting antidepressants. The data also identified unique prefrontal and striatal circuitry as a putative marker of successful treatment and a target for antidepressants' development.
Figures
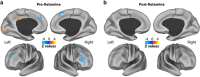
Figure 1
GBCr alterations in MDD at baseline and following ketamine treatment. Clusters mark brain regions with significant GBCr reduction (blue) or increase (red–yellow) in MDD compared with HC prior to ketamine treatment (a) and 24 h after intravenous infusion of ketamine (b) (whole-brain voxel-wise independent _t_-test with corrected α<0.05). The prefrontal cortex region is labeled with a black line. HC, healthy controls; GBCr, global brain connectivity with global signal regression; MDD, major depressive disorder. A full color version of this figure is available at the Neuropsychopharmacology journal online.
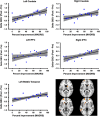
Figure 2
Individual variation in delta GBCr. This figure depict the spread at the subject level of both the percentage of improvement of depressive symptoms and the average GBCr changes in each of the five clusters identified in the responders vs non-responders analysis. The gray area is the 95% confidence band of the best-fit line. Clusters' locations are shown in right lower panel. GBCr, global brain connectivity with global signal regression; lPFC, lateral prefrontal cortex; MADRS, Montgomery–Åsberg Depression Rating Scale.
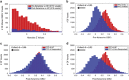
Figure 3
Distributions of GBCr at baseline and following ketamine treatment. (a) Distributions of absolute _z_-values of voxels showing significant reduction or increase of GBCr in MDD compared with HC prior to ketamine treatment (red) or 24 h after intravenous infusion of ketamine (blue). Although both distributions largely overlapped, the numbers of altered GBCr were numerically higher preketamine in each bin of the absolute _z_-values up to _z_~3.5. Of the 310 significant voxels postketamine, 123 were altered at baseline and 187 differed between groups only at the 24 h time point. (b) Distributions of GBCr in the anatomically defined PFC (delineated in Figure 1) in MDD and HC prior to ketamine treatment. The histograms depict comparable normal distributions in both groups with large effect size left shift in MDD reflecting overall reduction in PFC GBCr. (c and d) Distributions of PFC GBCr in HC and MDD responders (c) or MDD non-responders (d) 24 h after infusion of ketamine. The histograms reveal large overlap between the PFC GBCr distributions of HC and MDD responders (c). However, MDD non-responders continued to show large effect size left shift, suggesting a lack of normalization of PFC GBCr in this subgroup. GBCr, global brain connectivity with global signal regression; HC, healthy controls; MDD, major depressive disorder; PFC, prefrontal cortex. A full color version of this figure is available at the Neuropsychopharmacology journal online.
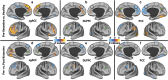
Figure 4
Seed-based connectivity alteration in the affective (AN), cognitive Control (CCN), and default mode networks (DMN). (a–c): Clusters mark brain regions with significant connectivity reduction (blue) or increase (red–yellow) in MDD compared with HC at baseline. (d–f) Clusters mark brain regions with significant connectivity reduction (blue) or increase (red–yellow) in following ketamine treatment. The prefrontal cortex region is labeled with a black line. The seeds' locations are marked with green circles. The empty black circles locate the dorsal nexus area. DLPFC, dorsolateral prefrontal cortex; HC, healthy controls; MDD, major depressive disorder; PCC, posterior cingulate and precuneus area; sgACC, subgenual anterior cingulate cortex. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Similar articles
- Ketamine, but Not the NMDAR Antagonist Lanicemine, Increases Prefrontal Global Connectivity in Depressed Patients.
Abdallah CG, Dutta A, Averill CL, McKie S, Akiki TJ, Averill LA, Deakin JFW. Abdallah CG, et al. Chronic Stress (Thousand Oaks). 2018 Jan-Dec;2:2470547018796102. doi: 10.1177/2470547018796102. Epub 2018 Sep 21. Chronic Stress (Thousand Oaks). 2018. PMID: 30263977 Free PMC article. - Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration.
Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA Jr. Evans JW, et al. Biol Psychiatry. 2018 Oct 15;84(8):582-590. doi: 10.1016/j.biopsych.2018.01.027. Epub 2018 Feb 15. Biol Psychiatry. 2018. PMID: 29580569 Free PMC article. Clinical Trial. - Habenula Connectivity and Intravenous Ketamine in Treatment-Resistant Depression.
Rivas-Grajales AM, Salas R, Robinson ME, Qi K, Murrough JW, Mathew SJ. Rivas-Grajales AM, et al. Int J Neuropsychopharmacol. 2021 May 18;24(5):383-391. doi: 10.1093/ijnp/pyaa089. Int J Neuropsychopharmacol. 2021. PMID: 33249434 Free PMC article. Clinical Trial. - Chronic stress pathology and ketamine-induced alterations in functional connectivity in major depressive disorder: An abridged review of the clinical evidence.
Averill LA, Fouda S, Murrough JW, Abdallah CG. Averill LA, et al. Adv Pharmacol. 2020;89:163-194. doi: 10.1016/bs.apha.2020.04.003. Epub 2020 May 14. Adv Pharmacol. 2020. PMID: 32616206 Review. - Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature.
Ionescu DF, Felicione JM, Gosai A, Cusin C, Shin P, Shapero BG, Deckersbach T. Ionescu DF, et al. Harv Rev Psychiatry. 2018 Nov/Dec;26(6):320-339. doi: 10.1097/HRP.0000000000000179. Harv Rev Psychiatry. 2018. PMID: 29465479 Free PMC article. Review.
Cited by
- Common and Specific Alterations of Amygdala Subregions in Major Depressive Disorder With and Without Anxiety: A Combined Structural and Resting-State Functional MRI Study.
Li YY, Ni XK, You YF, Qing YH, Wang PR, Yao JS, Ren KM, Zhang L, Liu ZW, Song TJ, Wang J, Zang YF, Shen YD, Chen W. Li YY, et al. Front Hum Neurosci. 2021 Feb 15;15:634113. doi: 10.3389/fnhum.2021.634113. eCollection 2021. Front Hum Neurosci. 2021. PMID: 33658914 Free PMC article. - Children with developmental coordination disorder show altered functional connectivity compared to peers.
Rinat S, Izadi-Najafabadi S, Zwicker JG. Rinat S, et al. Neuroimage Clin. 2020;27:102309. doi: 10.1016/j.nicl.2020.102309. Epub 2020 Jun 12. Neuroimage Clin. 2020. PMID: 32590334 Free PMC article. - Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model.
Price RB, Duman R. Price RB, et al. Mol Psychiatry. 2020 Mar;25(3):530-543. doi: 10.1038/s41380-019-0615-x. Epub 2019 Dec 4. Mol Psychiatry. 2020. PMID: 31801966 Free PMC article. Review. - Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments.
Duman RS, Sanacora G, Krystal JH. Duman RS, et al. Neuron. 2019 Apr 3;102(1):75-90. doi: 10.1016/j.neuron.2019.03.013. Neuron. 2019. PMID: 30946828 Free PMC article. Review. - Global Functional Connectivity Analysis Indicating Dysconnectivity of the Hate Circuit in Major Depressive Disorder.
Pan P, Wang L, Wu C, Jin K, Cao S, Qiu Y, Teng Z, Li S, Shao T, Huang J, Wu H, Xiang H, Chen J, Liu F, Tang H, Guo W. Pan P, et al. Front Aging Neurosci. 2022 Feb 17;13:803080. doi: 10.3389/fnagi.2021.803080. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35250533 Free PMC article.
References
- Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y et al (2015. a). N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77: 569–580. - PubMed
MeSH terms
Substances
Grants and funding
- K23 MH094707/MH/NIMH NIH HHS/United States
- K23 MH101498/MH/NIMH NIH HHS/United States
- UL1 TR000067/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous