α-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease - PubMed (original) (raw)
. 2016 Jun 16;3(8):588-606.
doi: 10.1002/acn3.321. eCollection 2016 Aug.
Affiliations
- PMID: 27606342
- PMCID: PMC4999592
- DOI: 10.1002/acn3.321
α-synuclein conformational antibodies fused to penetratin are effective in models of Lewy body disease
Brian Spencer et al. Ann Clin Transl Neurol. 2016.
Abstract
Objective: Progressive accumulation of α-synuclein (α-syn) has been associated with Parkinson's disease (PD) and Dementia with Lewy body (DLB). The mechanisms through which α-syn leads to neurodegeneration are not completely clear; however, the formation of various oligomeric species have been proposed to play a role. Antibody therapy has shown effectiveness at reducing α-syn accumulation in the central nervous system (CNS); however, most of these studies have been conducted utilizing antibodies that recognize both monomeric and higher molecular weight α-syn. In this context, the main objective of this study was to investigate the efficacy of immunotherapy with single-chain antibodies (scFVs) against specific conformational forms of α-syn fused to a novel brain penetrating sequence.
Method: We screened various scFVs against α-syn expressed from lentiviral vectors by intracerebral injections in an α-syn tg model. The most effective scFVs were fused to the cell-penetrating peptide penetratin to enhance transport across the blood-brain barrier, and lentiviral vectors were constructed and tested for efficacy following systemic delivery intraperitoneal into α-syn tg mice.
Result: Two scFVs (D5 and 10H) selectively targeted different α-syn oligomers and reduced the accumulation of α-syn and ameliorated functional deficits when delivered late in disease development; however, only one of the antibodies (D5) was also effective when delivered early in disease development. These scFVs were also utilized in an enzyme-linked immunosorbent assay (ELISA) assay to monitor the effects of immunotherapy on α-syn oligomers in brain and plasma.
Interpretation: The design and targeting of antibodies for specific species of α-syn oligomers is crucial for therapeutic immunotherapy and might be of relevance for the treatment of Lewy body disease.
Figures
Figure 1
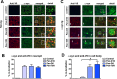
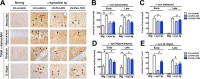
Double immunocytochemical analysis with scFvs against _α_‐syn detect _α_‐syn aggregates in the brains of a tg mouse. Brain vibratome sections from 6‐month‐old (A) non‐tg and (B) _α_‐syn tg mice were double labeled with antibodies against _α_‐syn (detected with a secondary
FITC
tagged) and the scFvs Pen‐D10, Pen‐D5, or Pen‐10H followed by Anti‐V5 (detected with Tyramide Red) and imaged with the laser scanning confocal microscope. Images are from the frontotemporal cortex. The sc
FV
s as detected with the antibody against the V5 tag colocalized with _α_‐syn in the brains of tg mice. Open boxes (white margins) in the merged panels indicate areas of higher magnification in detail panels. Arrows indicate intraneuronal _α_‐syn inclusions. (C) Computer aided image analysis of the % of Anti‐V5 (sc
FV
) colocalizing with total neuropil associated _α_‐syn immunofluorescence in the fronto‐temporal cortex. (D) Computer aided image analysis of the % of Anti‐V5 (sc
FV
) colocalizing with intracellular _α_‐syn immunofluorescence in the fronto‐temporal cortex. (n = 4, mice per group) #P < 0.05 when comparing _α_‐syn tg treated with lentivirus (
LV
)‐control vs
LV
‐sc
FV
s by one‐way
ANOVA
post hoc Tukey–Kramer. Scale bar represents 10 _μ_m.
Figure 2
In vitro analysis of the effects of the Pen‐scFvs against _α_‐syn expressed from lentivirus vectors reducing the accumulation of _α_‐syn in a neurons in a chamber system. (A) Diagrammatic representation of experiment depicting the B103 neuronal cells and coinfection with lentivirus (
LV
)‐_α_‐syn and
LV
‐Pen‐D10,
LV
‐Pen‐D5 or
LV
‐Pen‐10H for 48 h and then evaluated by immunocytochemistry for levels of _α_‐syn and sc
FV
s (V5 tag). (B) Coverslips were fixed and double immunostained with antibodies against _α_‐syn (red) and the scFv (V5 epitope tag, green) and imaged with the laser scanning confocal microscope. (C) Relative fluorescence was analyzed to determine levels of _α_‐syn and scFv immunoreactivity expressed as pixel intensity. (D) Diagrammatic representation of experiment depicting the B103 neuronal cells that were cocultured with cells in the lower chamber on coverslips infected with
LV
‐_α_‐syn and cells in the upper chamber infected with
LV
‐Pen‐D10,
LV
‐Pen‐D5 or
LV
‐Pen‐10H for 48 h. Chambers were separated by a 0.22 _μ_m membrane to allow for the passage of only proteins and small molecules. (E) Coverslips were fixed and double immunostained with antibodies against _α_‐syn (red) and the scFv (V5 epitope tag, green). (F) Relative fluorescence was analyzed to determine levels of _α_‐syn and scFv immunoreactivity expressed as pixel intensity. *Indicates statistical significance P < 0.05 compared to cell expressing _α_‐syn and treated with
LV
‐control. Experiments were performed in triplicate. One‐way
ANOVA
with post hoc Tukey–Kramer. Scale bar represents 10 _μ_m.
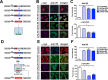
Figure 3
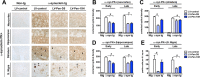
Immunocytochemical analysis of the effects of the Pen‐scFvs against morphologically distinct _α_‐syn species following stereotaxic intracerebral injection of lentivirus vectors in _α_‐syn tg mouse. Six‐month‐old _α_‐syn tg and non‐tg mice received hippocampal injections of lentivirus (
LV
) and were sacrificed 6 weeks later and serial sections prepared. (A) Immunocytochemical analysis with an antibody against the V5 tag (to detect scFvs) and (B) _α_‐syn in brain sections from non‐tg and _α_‐syn tg mice that received a unilateral stereotaxic of
LV
‐control,
LV
‐Pen‐D10,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Sections were analyzed with digital bright field video microscope, panels in the top represent an overview at low magnification (20X). Panels in the bottom are higher magnification (400X) of the hippocampus at the injection site. (C) Image analysis of levels expressed as corrected optical density for anti‐V5 immunoreactivity and (D) anti‐_α_‐syn immunoreactivity in the hippocampus, respectively. *Indicates statistical significance P < 0.05 compared to non‐tg to _α_‐syn tg treated with
LV
‐control by one‐way
ANOVA
with post hoc Dunnet's. #P < 0.05 when comparing _α_‐syn tg treated with
LV
‐control vs
LV
‐sc
FV
s by one‐way
ANOVA
post hoc Tukey–Kramer. Scale bar in upper panels represents 250 _μ_m and in lower panel 25 _μ_m.
Figure 4
Immunocytochemical analysis of levels of
CNS
accumulation of Pen‐scFv antibody (V5) following systemic delivery of a lentivirus vector expressing Pen‐scFvs against _α_‐syn. The non‐tg and _α_‐syn tg mice (early and late groups) received a single intraperitoneal injection of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Three months after injection, mice were sacrificed and serial sections were prepared. (A) Sections were immunostained with antibodies against V5 to identify the scFv. Upper panels are an overview of the brain sections at low magnification (20X), the lower panels are higher magnification (400X) areas (dotted squares) of the neocortex and hippocampus. Arrows indicate neuronal immunostaining. (B) Image analysis of levels of scFv immunoreactivity (V5 immunoreactivity) expressed as corrected optical density from early group (3 month old at start and 6 month old at the end of the experiment) mice. (C) Image analysis of levels of scFv immunoreactivity (V5 immunoreactivity) expressed as corrected optical density of sections from late group (6–9 month old at start and 9–12 month old at the end of the experiment) mice. *Indicates statistical significance P < 0.05 compared to non‐tg mice. One‐way
ANOVA
with post hoc Tukey–Kramer. Scale bar represents 200 _μ_m in low power images and 40 _μ_m in high power images.
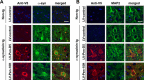
Figure 5
The Pen‐sc
FV
antibodies colocalize with _α_‐synuclein and neurons in the brain. Vibratome brain sections from non‐tg and _α_‐syn tg that received i.p. injections of lentivirus (
LV
)‐control or
LV
‐Pen‐D5 or
LV
‐Pen‐10H were double immunofluorescence labeled with antibodies against human _α_‐syn or
MAP
2 and the Pen‐sc
FV
(V5) and analyzed with the laser scanning confocal microscope. Representative neurons from the frontal cortex are presented, the panels in red corresponds to the sc
FV
(anti‐V5 antibody) and in green to the _α_‐syn or
MAP
2. (A) Double immunolabeling for the Pen‐sc
FV
(V5) (red) and human _α_‐syn (green) with nuclei (
DAPI
, blue) showing colocalization between the two markers in tg mice treated with Pen‐D5 and Pen‐10H but not with the control. (B) Double immunolabeling for the Pen‐sc
FV
(V5) (red) and the neuronal marker
MAP
2 (green) with nuclei (
DAPI
, blue). In tg mice treated with Pen‐D5 or Pen‐10H the sc
FV
colocalizes to
MAP
2‐positive neurons. Scale bar = 10 _μ_m.
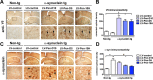
Figure 6
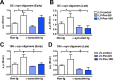
Systemic delivery of lentivirus vector expressing Pen‐scFvs against _α_‐syn reduces
CNS
accumulation of total _α_‐syn. The non‐tg and _α_‐syn tg mice (early and late groups) received a single intraperitoneal injection of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Three months after injection, mice were sacrificed and serial sections were prepared for immunocytochemical analysis with an antibody against total _α_‐syn and imaged with a bright field digital video microscope. (A) Representative images of the neocortex, hippocampus, striatum and substantia nigra of the early (3 months old at start) group of non‐tg and _α_‐syn tg mice immunostained with an antibody against _α_‐syn. (n) neuropil (arrows) neuronal cell bodies. Image analysis of levels of _α_‐syn immunoreactivity expressed as corrected optical density of sections from early (3 month old) mice and late (6–9 month old) mice in the (B) neocortex, (C) striatum, (D) hippocampus, and (E) substantia nigra. *Indicates statistical significance P < 0.05 compared to non‐tg mice. #Indicates statistical significance P < 0.05 compared to _α_‐syn tg mice treated with
LV
‐control. One‐way
ANOVA
with post hoc Tukey–Kramer. Scale bar represents 40 _μ_m in high power images.
Figure 7
Effects of systemically delivered lentivirus vectors expressing Pen‐scFvs against _α_‐syn on the accumulation of proteinase K (
PK
)‐resistant _α_‐syn in the
CNS
. The non‐tg and _α_‐syn tg mice (early and late groups) received a single intraperitoneal injection of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Three months after injection, mice were sacrificed and serial sections were pretreated with
PK
followed by immunocytochemical analysis with an antibody against total _α_‐syn and imaged with a bright field digital video microscope. (A) Representative images of the neocortex, hippocampus, striatum and substantia nigra of the early (3 month old at start) group of non‐tg and _α_‐syn tg mice. (arrowheads) dystrophic neurites and (arrows) neuronal cell bodies. (B) Image analysis of levels of
PK
‐resistant _α_‐syn immunoreactivity expressed as corrected optical density of sections from early (3 month old) mice and late (6–9 month old) mice in the (B) neocortex, (C) striatum, (D) hippocampus, and (E) substantia nigra. #Indicates statistical significance P < 0.05 compared to _α_‐syn tg mice treated with
LV
‐control. One‐way
ANOVA
with post hoc Tukey–Kramer. Scale bar represents 40 _μ_m in high power images.
Figure 8
Analysis by
ELISA
of the levels of distinct _α_‐syn oligomeric species in the
CNS
of mice following systemic treatment with Pen‐scFv against _α_‐syn. _α_‐syn tg or non‐tg mice received a single intraperitoneal injection of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Three months after injection, mice were sacrificed and whole brain homogenates were prepared and analyzed by
ELISA
. The D5 scFv was used as a capture antibody to assay (A) early (3 month old) mice and (B) late (6–9 month old) mice. The 10H scFv was used as a capture antibody to assay (C) early (3 month old) mice and (D) late (6–9 month old) mice. *Indicates statistical significance P < 0.05 compared to non‐tg mice. #Indicates statistical significance P < 0.05 compared to _α_‐syn tg mice treated with
LV
‐control. One‐way
ANOVA
with post hoc Tukey–Kramer.
Figure 9
Effects of systemic delivered lentivirus vectors expressing Pen‐scFvs against _α_‐syn on neuronal and astroglial pathology in a _α_‐syn tg mouse. The _α_‐syn tg and non‐tg mice received intraperitoneal injections of lentivirus (
LV
)‐Pen‐D5,
LV
‐Pen‐10H or
LV
‐control. Three months after injection, mice were sacrificed and brain sections were immunostained with antibodies against the neuronal (NeuN) and astroglial cell glial fibrillary acidic protein (
GFAP
) markers and imaged with a bright field digital video microscope. (A) Representative images from early group (3 month old at start) non‐tg and _α_‐syn tg mice immunostained with antibodies against the neuronal marker NeuN. The upper panel is a low magnification (20X) overview, while the lower power is a higher magnification (400X) view of the field in the hippocampus (
CA
- marked with a solid line rectangle. Stereological estimates (dissector method) of total NeuN‐positive neuronal counts measured in the (B) frontal cortex and (C) hippocampus of early (3 month old at start) and late (6–9 month old at start) mice. (D) Representative images from early group (3 month old at start) of non‐tg and _α_‐syn tg mice immunostained with antibodies against the astroglial marker
GFAP
. The upper panel is a low magnification (20X) overview, whereas the lower power is a higher magnification (400X) view of the field in the hippocampus (
CA
- marked with a solid line rectangle. Image analysis of levels of
GFAP
immunoreactivity expressed as corrected optical density of sections from the (E) frontal cortex and (F) hippocampus. *Indicates statistical significance P < 0.05 compared to non‐tg mice. #Indicates statistical significance P < 0.05 compared to _α_‐syn tg mice treated with
LV
‐control. One‐way
ANOVA
with post hoc Tukey–Kramer. Scale bar represents 200 _μ_m in low power images and 40 _μ_m in high power images.
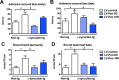
Figure 10
Effects of systemic treatment with a lentivirus vector expressing Pen‐scFvs against _α_‐syn at ameliorating motor deficits in _α_‐syn tg mice. The _α_‐syn tg and non‐tg received a single intraperitoneal injections of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H and 3 months later, motor coordination was assessed with the adhesive removal test. (A) Early group (3 month old at start) mice were examined at the end of the treatment by placing a small adhesive sticker on their nose and the time to removal was recorded. Compared to
LV
‐control, non‐tg mice, the _α_‐syn tg mice displayed delayed removal of the adhesive that was improved by the treatment with the
LV
‐Pen‐D5. (B) Late group (6–9 month old at start) mice were examined similarly at the end of the treatment. Compared to
LV
‐control non‐tg mice, the _α_‐syn tg mice displayed delayed removal of the adhesive that was improved by the treatment with the
LV
‐Pen‐10H. Gait and balance were assessed with the round beam. (C) Early group mice were examined at the end of the treatment and assessed for number of missed foot placements on the beam as errors/10 cm. (D) Late group mice were examined at the end of treatment and assessed for the number of foot placement errors as errors/10 cm. *Indicates statistical significance P < 0.05 when compared with non‐tg controls. #Indicates statistical significance P < 0.05 when compared to _α_‐syn tg mice that received
LV
‐control. One‐way
ANOVA
with post hoc Dunnet's.
Figure 11
The sc
FV
s antibodies against distinct _α_‐syn oligomers can be detected by _α_‐syn
ELISA
in the serum and monitor treatment effect. The _α_‐syn tg or non‐tg mice received a single intraperitoneal injection of lentivirus (
LV
)‐control,
LV
‐Pen‐D5 or
LV
‐Pen‐10H. Three months after injection, serum was collected and assayed by
ELISA
using the scFvs as capture antibodies. The D5 scFv was used as a capture antibody to assay (A) early (3 month old) mice and (B) late (6–9 month old) mice. The 10H scFv was used as a capture antibody to assay (C) early (3 month old) mice and (D) late (6–9 month old) mice. *Indicates statistical significance P < 0.05 compared to non‐tg mice. #Indicates statistical significance P < 0.05 compared to _α_‐syn tg mice treated with
LV
‐control. One‐way
ANOVA
with post hoc Tukey–Kramer.
Similar articles
- Anti-α-synuclein immunotherapy reduces α-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy.
Spencer B, Valera E, Rockenstein E, Overk C, Mante M, Adame A, Zago W, Seubert P, Barbour R, Schenk D, Games D, Rissman RA, Masliah E. Spencer B, et al. Acta Neuropathol Commun. 2017 Jan 13;5(1):7. doi: 10.1186/s40478-016-0410-8. Acta Neuropathol Commun. 2017. PMID: 28086964 Free PMC article. - Longitudinal live imaging of retinal α-synuclein::GFP deposits in a transgenic mouse model of Parkinson's Disease/Dementia with Lewy Bodies.
Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, Duong-Polk KX, Bonhaus D, Lindsey J, Masliah E. Price DL, et al. Sci Rep. 2016 Jul 8;6:29523. doi: 10.1038/srep29523. Sci Rep. 2016. PMID: 27389831 Free PMC article. - Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson's disease-like models.
Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, Zago W, Seubert P, Barbour R, Schenk D, Masliah E. Games D, et al. J Neurosci. 2014 Jul 9;34(28):9441-54. doi: 10.1523/JNEUROSCI.5314-13.2014. J Neurosci. 2014. PMID: 25009275 Free PMC article. - Neuropathology of synuclein aggregates.
Duda JE, Lee VM, Trojanowski JQ. Duda JE, et al. J Neurosci Res. 2000 Jul 15;61(2):121-7. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. J Neurosci Res. 2000. PMID: 10878583 Review. - Immunotherapy targeting α-synuclein, with relevance for future treatment of Parkinson's disease and other Lewy body disorders.
Lindström V, Ihse E, Fagerqvist T, Bergström J, Nordström E, Möller C, Lannfelt L, Ingelsson M. Lindström V, et al. Immunotherapy. 2014;6(2):141-53. doi: 10.2217/imt.13.162. Immunotherapy. 2014. PMID: 24491088 Review.
Cited by
- Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson's Disease and Other Lewy Body Disorders.
Ingelsson M. Ingelsson M. Front Neurosci. 2016 Sep 5;10:408. doi: 10.3389/fnins.2016.00408. eCollection 2016. Front Neurosci. 2016. PMID: 27656123 Free PMC article. Review. - Characterization of novel conformation-selective α-synuclein antibodies as potential immunotherapeutic agents for Parkinson's disease.
Henderson MX, Covell DJ, Chung CH, Pitkin RM, Sandler RM, Decker SC, Riddle DM, Zhang B, Gathagan RJ, James MJ, Trojanowski JQ, Brunden KR, Lee VMY, Luk KC. Henderson MX, et al. Neurobiol Dis. 2020 Mar;136:104712. doi: 10.1016/j.nbd.2019.104712. Epub 2019 Dec 16. Neurobiol Dis. 2020. PMID: 31837422 Free PMC article. - Transportation of Single-Domain Antibodies through the Blood-Brain Barrier.
Ruiz-López E, Schuhmacher AJ. Ruiz-López E, et al. Biomolecules. 2021 Jul 31;11(8):1131. doi: 10.3390/biom11081131. Biomolecules. 2021. PMID: 34439797 Free PMC article. Review. - Antibody Fragments as Tools for Elucidating Structure-Toxicity Relationships and for Diagnostic/Therapeutic Targeting of Neurotoxic Amyloid Oligomers.
Bitencourt ALB, Campos RM, Cline EN, Klein WL, Sebollela A. Bitencourt ALB, et al. Int J Mol Sci. 2020 Nov 24;21(23):8920. doi: 10.3390/ijms21238920. Int J Mol Sci. 2020. PMID: 33255488 Free PMC article. Review. - Fibrillar form of α-synuclein-specific scFv antibody inhibits α-synuclein seeds induced aggregation and toxicity.
Gupta V, Salim S, Hmila I, Vaikath NN, Sudhakaran IP, Ghanem SS, Majbour NK, Abdulla SA, Emara MM, Abdesselem HB, Lukacsovich T, Erskine D, El-Agnaf OMA. Gupta V, et al. Sci Rep. 2020 May 18;10(1):8137. doi: 10.1038/s41598-020-65035-8. Sci Rep. 2020. PMID: 32424162 Free PMC article.
References
- Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha‐synuclein. Ann Neurol 1998;44:415–422. - PubMed
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha‐synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 1998;249:180–182. - PubMed
- Spillantini MG, Crowther RA, Jakes R, et al. Filamentous alpha‐synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 1998;251:205–208. - PubMed
- Zarranz JJ, Alegre J, Gomez‐Esteban JC, et al. The new mutation, E46K, of alpha‐synuclein causes Parkinson and Lewy body dementia. Ann Neurol 2004;55:164–173. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous