Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine - PubMed (original) (raw)
Review
Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine
Kimberly J Dunham-Snary et al. Chest. 2017 Jan.
Abstract
Hypoxic pulmonary vasoconstriction (HPV) is a homeostatic mechanism that is intrinsic to the pulmonary vasculature. Intrapulmonary arteries constrict in response to alveolar hypoxia, diverting blood to better-oxygenated lung segments, thereby optimizing ventilation/perfusion matching and systemic oxygen delivery. In response to alveolar hypoxia, a mitochondrial sensor dynamically changes reactive oxygen species and redox couples in pulmonary artery smooth muscle cells (PASMC). This inhibits potassium channels, depolarizes PASMC, activates voltage-gated calcium channels, and increases cytosolic calcium, causing vasoconstriction. Sustained hypoxia activates rho kinase, reinforcing vasoconstriction, and hypoxia-inducible factor (HIF)-1α, leading to adverse pulmonary vascular remodeling and pulmonary hypertension (PH). In the nonventilated fetal lung, HPV diverts blood to the systemic vasculature. After birth, HPV commonly occurs as a localized homeostatic response to focal pneumonia or atelectasis, which optimizes systemic Po2 without altering pulmonary artery pressure (PAP). In single-lung anesthesia, HPV reduces blood flow to the nonventilated lung, thereby facilitating thoracic surgery. At altitude, global hypoxia causes diffuse HPV, increases PAP, and initiates PH. Exaggerated or heterogeneous HPV contributes to high-altitude pulmonary edema. Conversely, impaired HPV, whether due to disease (eg, COPD, sepsis) or vasodilator drugs, promotes systemic hypoxemia. Genetic and epigenetic abnormalities of this oxygen-sensing pathway can trigger normoxic activation of HIF-1α and can promote abnormal metabolism and cell proliferation. The resulting pseudohypoxic state underlies the Warburg metabolic shift and contributes to the neoplasia-like phenotype of PH. HPV and oxygen sensing are important in human health and disease.
Keywords: chuvash polycythemia; high altitude pulmonary edema; mitochondria; oxygen-sensitive potassium channels; single-lung anesthesia; ventilation/perfusion matching.
Copyright © 2016 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
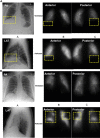
Figure 1
Matched vs mismatched ventilation/perfusion (V./Q.) defects. (A) Matched V./Q. defect due to interstitial lung disease. An 83-year-old man with shortness of breath on exertion. Chest CT showed interstitial lung disease with a basal predominance consistent with usual interstitial pneumonitis. (B) Mismatched defect indicating pulmonary embolism. A 57-year-old man with obesity, hypertension, and OSA presenting with shortness of breath and desaturation with minimal exertion. PA = posteroanterior; LAT = lateral.
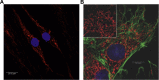
Figure 2
Pulmonary artery (PA) mitochondrial network. (A) Mitochondria in pulmonary artery smooth muscle cells from a normal patient were stained with 20 nM tetramethylrhodamine and imaged using confocal microscopy. Nuclei stained with NucBlue Live Cell Stain. (B) Bovine pulmonary artery (PA) endothelial cells stained for mitochondria (inset: red, MitoTracker Red CMXRos), phalloidin (green, Alexa Fluor 488), and nucleus (blue, 4′,6-diamidino-2-phenylindole) and imaged using confocal microscopy. All dyes/stains from Life Technologies (Carlsbad, CA).
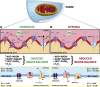
Figure 3
Mitochondrial redox oxygen sensing. The sensor-effector mechanism of hypoxic pulmonary vasoconstriction (HPV). (A) Under normoxic conditions, generation of reactive oxygen species (ROS) occurs at mitochondrial electron transport chain (ETC) complexes I and III, producing superoxide (O2–), which is converted to hydrogen peroxide (H2O2) by superoxide dismutase 2 (SOD2). Hydrogen peroxide, along with the oxidized redox couples (eg, nicotinamide adenine dinucleotide [NAD+], nicotinamide adenine dinucleotide phosphate [NADP+], and flavin adenine dinucleotide [FAD2+]) maintain Kv1.5 sulfhydryl group oxidation and channel open state, resulting in tonic egress of K+. This efflux of K+ sustains the resting membrane potential (ΔΨ) of the cell at –60 mV and inhibits voltage-gated calcium channel [CaL]-mediated Ca2+ influx into the cell. (B) During hypoxia, the limited presence of oxygen (1) prevents generation of hydrogen peroxide, (2) decreases the ratio of oxidized/reduced redox couples, and (3) reduces sulfhydryl groups on Kv1.5 channels, causing them to close. The subsequent buildup of K+ increases the resting membrane potential of the cell to –20 mV. This stimulates the opening of CaL, influx of Ca2+, and subsequent activation of the contractile apparatus (ie, vasoconstriction). ADP = adenosine diphosphate; FADH2 = flavin adenine dinucleotide; NADH = nicotinamide adenine dinucleotide.
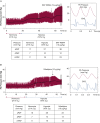
Figure 4
Voltage-gated calcium channels [CaL] and hypoxic pulmonary vasoconstriction (HPV). CaL agonist BAY K8644 and antagonist nifedipine exert opposing effects on HPV in the anesthetized hypoxic rat ventilated with 10% oxygen. This model of global hypoxia mimics changes that occur with ascent to altitude and results in a rapid increase in pulmonary artery pressure (PAP). (A) BAY K8644 enhances PA vasoconstriction during hypoxia. (B) Nifedipine administered during HPV inhibits CaL and reduces PAP. dPAP = diastolic PAP; ECG = electrocardiography; mPAP = mean PAP; sPAP = systolic PAP.
Figure 5
Clinical application of hypoxic pulmonary vasoconstriction (HPV). Maintenance of P
o
2 during thoracic surgery through single-lung ventilation. (A) A Broncho-Cath double-lumen endotracheal tube (Covidien, Saint-Laurent, QC). The distal end (orange arrow) is inserted into the trachea until the bronchial lumen (inset, blue arrow) has entered either the right or left mainstem bronchus, whereas the tracheal lumen (inset, purple arrow) remains above the carina. (B) A bronchoscopic view of the carina from inside the tracheal lumen. The distal tip of the double-lumen endotracheal tube is seen entering the left mainstem bronchus to enable single-lung ventilation. (C) A clamp is placed on one limb of the ventilation circuit, allowing selective ventilation of the opposite lung. (D) Cardiorespiratory monitor during single-lung anesthesia/ventilation. The end tidal CO2 is elevated to 53 mm Hg because of the decreased ventilation (red arrow), whereas oxygen (O2) saturation is maintained at 99% (green arrow) and blood pressure (BP) remains stable (orange arrow) despite single-lung ventilation. (E) The nonventilated deflated lung undergoing surgery can be seen through an incision. Minimal bleeding is seen during surgery as a result of the HPV response. (F) Pneumonectomy specimen showing the complete lung removed with a large tumor (yellow box).
Similar articles
- Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells.
Michelakis ED, Thébaud B, Weir EK, Archer SL. Michelakis ED, et al. J Mol Cell Cardiol. 2004 Dec;37(6):1119-36. doi: 10.1016/j.yjmcc.2004.09.007. J Mol Cell Cardiol. 2004. PMID: 15572043 Review. - Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation.
Archer SL, Weir EK, Reeve HL, Michelakis E. Archer SL, et al. Adv Exp Med Biol. 2000;475:219-40. doi: 10.1007/0-306-46825-5_21. Adv Exp Med Biol. 2000. PMID: 10849663 Review. - An anesthesiologist's guide to hypoxic pulmonary vasoconstriction: implications for managing single-lung anesthesia and atelectasis.
Nagendran J, Stewart K, Hoskinson M, Archer SL. Nagendran J, et al. Curr Opin Anaesthesiol. 2006 Feb;19(1):34-43. doi: 10.1097/01.aco.0000192777.09527.9e. Curr Opin Anaesthesiol. 2006. PMID: 16547431 Review. - A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus.
Dunham-Snary KJ, Hong ZG, Xiong PY, Del Paggio JC, Herr JE, Johri AM, Archer SL. Dunham-Snary KJ, et al. Pflugers Arch. 2016 Jan;468(1):43-58. doi: 10.1007/s00424-015-1736-y. Epub 2015 Sep 23. Pflugers Arch. 2016. PMID: 26395471 Free PMC article. Review. - Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction.
Dunham-Snary KJ, Wu D, Potus F, Sykes EA, Mewburn JD, Charles RL, Eaton P, Sultanian RA, Archer SL. Dunham-Snary KJ, et al. Circ Res. 2019 Jun 7;124(12):1727-1746. doi: 10.1161/CIRCRESAHA.118.314284. Epub 2019 Mar 29. Circ Res. 2019. PMID: 30922174 Free PMC article.
Cited by
- mTOR in the Development of Hypoxic Pulmonary Hypertension Associated with Cardiometabolic Risk Factors.
Flores K, Almeida C, Arriaza K, Pena E, El Alam S. Flores K, et al. Int J Mol Sci. 2024 Oct 14;25(20):11023. doi: 10.3390/ijms252011023. Int J Mol Sci. 2024. PMID: 39456805 Free PMC article. Review. - New Insights Into Heat Shock Protein 90 in the Pathogenesis of Pulmonary Arterial Hypertension.
Hu L, Zhao R, Liu Q, Li Q. Hu L, et al. Front Physiol. 2020 Sep 15;11:1081. doi: 10.3389/fphys.2020.01081. eCollection 2020. Front Physiol. 2020. PMID: 33041844 Free PMC article. Review. - Can Hyperperfusion of Nonaerated Lung Explain COVID-19 Hypoxia?
Herrmann J, Mori V, Bates JHT, Suki B. Herrmann J, et al. Res Sq [Preprint]. 2020 Jun 1:rs.3.rs-32949. doi: 10.21203/rs.3.rs-32949/v1. Res Sq. 2020. PMID: 32702716 Free PMC article. Updated. Preprint. - Endothelial Extracellular Vesicles: From Keepers of Health to Messengers of Disease.
Mathiesen A, Hamilton T, Carter N, Brown M, McPheat W, Dobrian A. Mathiesen A, et al. Int J Mol Sci. 2021 Apr 28;22(9):4640. doi: 10.3390/ijms22094640. Int J Mol Sci. 2021. PMID: 33924982 Free PMC article. Review. - Effect of caspase inhibitors on hemodynamics and inflammatory factors in ARDS model rats.
Liu A, Tian F, Zhou Y, Pu Z. Liu A, et al. Sci Rep. 2024 Jul 15;14(1):16317. doi: 10.1038/s41598-024-67444-5. Sci Rep. 2024. PMID: 39009819 Free PMC article.
References
- Euler U.S.V., Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol Scand. 1946;12(4):301–320.
- Madden J.A., Dawson C.A., Harder D.R. Hypoxia-induced activation in small isolated pulmonary arteries from the cat. J Appl Physiol (1985) 1985;59(1):113–118. - PubMed
- Baraka A.S., Taha S.K., Yaacoub C.I. Alarming hypoxemia during one-lung ventilation in a patient with respiratory bronchiolitis-associated interstitial lung disease. Can J Cardiol. 2003;50(4):411–414. - PubMed
- Jensen K.S., Micco A.J., Czartolomna J., Latham L., Voelkel N.F. Rapid onset of hypoxic vasoconstriction in isolated lungs. J Appl Physiol (1985) 1992;72(5):2018–2023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials