Dynamics of Escherichia coli's passive response to a sudden decrease in external osmolarity - PubMed (original) (raw)
Dynamics of Escherichia coli's passive response to a sudden decrease in external osmolarity
Renata Buda et al. Proc Natl Acad Sci U S A. 2016.
Abstract
For most cells, a sudden decrease in external osmolarity results in fast water influx that can burst the cell. To survive, cells rely on the passive response of mechanosensitive channels, which open under increased membrane tension and allow the release of cytoplasmic solutes and water. Although the gating and the molecular structure of mechanosensitive channels found in Escherichia coli have been extensively studied, the overall dynamics of the whole cellular response remain poorly understood. Here, we characterize E. coli's passive response to a sudden hypoosmotic shock (downshock) on a single-cell level. We show that initial fast volume expansion is followed by a slow volume recovery that can end below the initial value. Similar response patterns were observed at downshocks of a wide range of magnitudes. Although wild-type cells adapted to osmotic downshocks and resumed growing, cells of a double-mutant ([Formula: see text]) strain expanded, but failed to fully recover, often lysing or not resuming growth at high osmotic downshocks. We propose a theoretical model to explain our observations by simulating mechanosensitive channels opening, and subsequent solute efflux and water flux. The model illustrates how solute efflux, driven by mechanical pressure and solute chemical potential, competes with water influx to reduce cellular osmotic pressure and allow volume recovery. Our work highlights the vital role of mechanosensation in bacterial survival.
Keywords: bacterial mechanosensing; osmotic downshock; single-cell imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
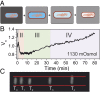
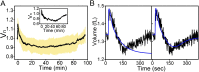
Characteristic cell volume response to a sudden downshock. (A) Upon a sudden decrease in external concentration, cell volume expands, which leads to opening of mechanosensitive channels. Consequently, solutes exit the cell, allowing recovery of cell volume through loss of cytoplasmic water. (B) A characteristic single-cell volume response for a 1,130-mOsmol downshock. The trace was normalized by the initial volume, that is, the volume before the downshock. Different phases of the recovery response are indicated with different colors. In gray is the expansion phase (phase I), followed by two volume recovery phases. Phase II (in orange) is characterized by volume decrease, and phase III (in green), by volume increase upon reaching the minimum volume. Phase IV (in purple) indicates recommenced growth. Initial 15 min are sampled at 5 Hz and an additional 1 h at a frame every 5 s. (C) Still images from different phases in B. T0 is the very beginning of the recording, before the downshock. T1 = 45 s, T2 = 4 min, T3 = 35 min, and T4 = 70 min. Red lines are drawn to indicate the size of the cell before the downshock. In comparison, the cell size at T1 is slightly larger (phase I), at T2 smaller (phase II), at T3 it reaches the initial size (phase III), and at T4 it is significantly larger (phase IV).
Fig. 2.
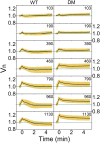
Cell volume response of the wild-type cells at different downshock magnitudes shows slow volume recovery and an “overshoot.” We use descriptive statistics to present our datasets and plot average volume traces with SDs constructed from 609 (WT) and 480 [double mutant (DM)] traces as a function of time. A zoom-in to first 5 min of downshock response sampled every 0.375 s is given. In total, 64 (WT) and 66 (DM) cells were used for 103 mOsmol, 94 (WT) and 52 (DM) cells were used for 190 mOsmol, 66 (WT) and 54 (DM) for 390 mOsmol, 56 (WT) and 80 (DM) for 460 mOsmol, 90 (WT) and 68 (DM) for 790 mOsmol, 116 (WT) and 50 (DM) for 960 mOsmol, and 106 (WT) and 77 (DM) cells for the 1,130-mOsmol downshock. Volume expands in all conditions and increases with the shock magnitude. Slow recovery follows volume expansion. For the wild-type cells, volume drops below the initial value, increasingly so with the larger shocks.
Fig. 3.
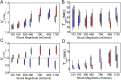
Analysis of maximum and minimum volume and time. (A) Box plot of maximum volume, Vn,max, and (B) the time at which maximum volume is reached, Tmax, as a function of shock magnitude. The wild type is shown in blue, and the double mutant, in red. The upper/lower whiskers indicate 1.5× the SD value. The upper/lower edges of the boxes indicate the third/first quartile. The black line indicates the median, and the yellow line, the average value. Vn,max increases with the shock magnitudes and saturates at and above 790 mOsmol. Tmax is independent from the shock magnitude for the wild type (blue) and slightly smaller for the double mutant (red) for the two largest shock magnitudes. (C) Vn,min and (D) Tmin plotted against the shock magnitude for wild type (blue) and double mutant (red). Vn,min in C is slightly below 1 for the wild type and decreases with the shock magnitude. Vn,min for the double mutant stays above 1 in all conditions. Tmin increases with the shock magnitude for both wild-type and double-mutant cells.
Fig. 4.
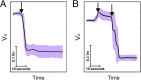
Cells, either grown at high osmolarities or subjected to a downshock, were exposed to a subsequent upshock. Normalized, average volume of (A) 13 and (B) 30 cells plotted against time in seconds. SD is given as a shaded area in light purple. (A) Cells grown in media of high osmolarity, 1,370 mOsmol, were subjected to a further increase of external osmolarity (upshock of 1,272 mOsmol). Arrow indicates the time at which shock was administered. Cell volume decreased within seconds posthyperosmotic shock. (B) Cells grown in the same media were subjected to a 1,130-mOsmol downshock indicated with the first arrow. Upon the downshock, volume expanded. At ≈ 1 min after the downshock, cells were exposed to a strong upshock of 2,160 mOsmol, indicated by the second arrow. Upon the upshock, cell volume decreased within seconds.
Fig. 5.
Model of cellular response to a sudden downshock. (A) A sudden decrease in external osmolarity leads to cell volume expansion and opening of mechanosensitive channels (panels 1 and 2). Upon channel opening, the water flux into the cell increases, as the water now flows inward through the channels as well (panel 3, blue arrow). Consequently, solutes exit the cell down the solute chemical potential and due to increased pressure inside the cell (panel 3, blue and red arrows). Solute efflux through the channels tips the competition between water influx and efflux toward efflux, which allows the recovery of cell volume to proceed (panel 4, blue arrow). (B) Cell volume (black), water influx (blue), water efflux (orange), and solute efflux (red) are given against time for the wild type (Left) and the double mutant (Right). All are solutions to the mathematical model equations using four free parameters obtained from the best fit to the representative cell volume trace in 960-mOsmol downshock condition. Eq. 10 was used to plot the cell volume. The first part of Eq. 10 was used for the water influx and the second part of Eq. 10 for the water efflux. Eq. 14 was used for solute efflux. Vertical gray lines indicate following events in sequential order: osmotic shock, opening of the mechanosensitive channels, the point in time when Vmax is reached, and closing of the channels. (C) Cell volume as predicted by the mathematical model given as a function of time. Parameters used in the best fit to the average cell volume in 960-mOsmol downshock condition were varied by ±100% for all parameters. Only one parameter is varied at a time, and the others are kept fixed. Green color indicates the lowest value used, and red, the highest (color scale is given on the Right). Increasing Vth increases Vmax, but lowers Vmin. Increasing α and A decreases Vmax but increases Vmin, whereas increasing K and ΔC0 increases Vmax and decreases Vmin, with a stronger effect on the Vmin reduction. E0l increase has little effect on Vmax, but it decreases Vmin. (D) Representative trace of the wild type (Top) and the double mutant (Bottom) for the 960-mOsmol condition is given in black. Blue line shows the result of best fit to the average trace. Shaded orange regions show fit confidence intervals; from lighter to darker orange, these are as follows: 50%, 90%, 95%, and 99%. There is a good agreement between the model and the experimental data.
Fig. 6.
Active response and postshock growth rates. (A) Black line shows average volume against time of 36 wild-type strains exposed to a 1,310-mOsmol downshock. Cells were grown in MM9 supplemented with NaCl and transferred into sodium phosphate buffer supplemented with 5 mM KCl. Shaded orange region indicates SD. Cell volume expanded and recovered, dropping below the initial volume. Within the last 30 min, volume increase is visible. Inset shows an example of an individual trace, where volume increase occurs after Vmin is reached at ≈ 60 min postdownshock. (B, Left) Wild-type representative trace (black) and the result of the global fit (blue) taken from Fig. 5_D_ and shown on a longer timescale. (Right) The fit (blue) is performed with the addition of the active pumping component (see SI Appendix for details on the extended model) and plotted against the same wild-type representative trace shown on the Left and in Fig. 5_D_ (black).
Similar articles
- Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time.
Boer M, Anishkin A, Sukharev S. Boer M, et al. Biochemistry. 2011 May 17;50(19):4087-96. doi: 10.1021/bi1019435. Epub 2011 Apr 20. Biochemistry. 2011. PMID: 21456519 Free PMC article. - Body shaping under water stress: osmosensing and osmoregulation of solute transport in bacteria.
Morbach S, Krämer R. Morbach S, et al. Chembiochem. 2002 May 3;3(5):384-97. doi: 10.1002/1439-7633(20020503)3:5<384::AID-CBIC384>3.0.CO;2-H. Chembiochem. 2002. PMID: 12007171 Review. - Gating the bacterial mechanosensitive channel MscL invivo.
Batiza AF, Kuo MM, Yoshimura K, Kung C. Batiza AF, et al. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5643-8. doi: 10.1073/pnas.082092599. Proc Natl Acad Sci U S A. 2002. PMID: 11960017 Free PMC article. - Managing hypoosmotic stress: aquaporins and mechanosensitive channels in Escherichia coli.
Booth IR, Louis P. Booth IR, et al. Curr Opin Microbiol. 1999 Apr;2(2):166-9. doi: 10.1016/s1369-5274(99)80029-0. Curr Opin Microbiol. 1999. PMID: 10322175 Review. - Plasmolysis and cell shape depend on solute outer-membrane permeability during hyperosmotic shock in E. coli.
Pilizota T, Shaevitz JW. Pilizota T, et al. Biophys J. 2013 Jun 18;104(12):2733-42. doi: 10.1016/j.bpj.2013.05.011. Biophys J. 2013. PMID: 23790382 Free PMC article.
Cited by
- Physicochemical homeostasis in bacteria.
Poolman B. Poolman B. FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad033. doi: 10.1093/femsre/fuad033. FEMS Microbiol Rev. 2023. PMID: 37336577 Free PMC article. - Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis.
Czech L, Hermann L, Stöveken N, Richter AA, Höppner A, Smits SHJ, Heider J, Bremer E. Czech L, et al. Genes (Basel). 2018 Mar 22;9(4):177. doi: 10.3390/genes9040177. Genes (Basel). 2018. PMID: 29565833 Free PMC article. Review. - Robust surface-to-mass coupling and turgor-dependent cell width determine bacterial dry-mass density.
Oldewurtel ER, Kitahara Y, van Teeffelen S. Oldewurtel ER, et al. Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2021416118. doi: 10.1073/pnas.2021416118. Proc Natl Acad Sci U S A. 2021. PMID: 34341116 Free PMC article. - arfA antisense RNA regulates MscL excretory activity.
Morra R, Pratama F, Butterfield T, Tomazetto G, Young K, Lopez R, Dixon N. Morra R, et al. Life Sci Alliance. 2023 Apr 3;6(6):e202301954. doi: 10.26508/lsa.202301954. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 37012050 Free PMC article. - Observing mechanosensitive channels in action in living bacteria.
Gh MS, Wilhelm MJ, Dai HL. Gh MS, et al. Biophys Rep (N Y). 2023 Dec 12;4(1):100141. doi: 10.1016/j.bpr.2023.100141. eCollection 2024 Mar 13. Biophys Rep (N Y). 2023. PMID: 38189030 Free PMC article.
References
- Koch AL. How bacteria grow and divide in spite of internal hydrostatic pressure. Can J Microbiol. 1985;31(12):1071–1084. - PubMed
- Deng Y, Sun M, Shaevitz JW. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett. 2011;107(15):158101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources