Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity - PubMed (original) (raw)
Molecular and Biological Compatibility with Host Alpha-Synuclein Influences Fibril Pathogenicity
Kelvin C Luk et al. Cell Rep. 2016.
Abstract
The accumulation and propagation of misfolded α-synuclein (α-Syn) is a central feature of Parkinson's disease and other synucleinopathies. Molecular compatibility between a fibrillar seed and its native protein state is a major determinant of amyloid self-replication. We show that cross-seeded aggregation of human (Hu) and mouse (Ms) α-Syn is bidirectionally restricted. Although fibrils formed by Hu-Ms-α-Syn chimeric mutants can overcome this inhibition in cell-free systems, sequence homology poorly predicts their efficiency in inducing α-Syn pathology in primary neurons or after intracerebral injection into wild-type mice. Chimeric α-Syn fibrils demonstrate enhanced or reduced pathogenicities compared with wild-type Hu- or Ms-α-Syn fibrils. Furthermore, α-Syn mutants induced to polymerize by fibrillar seeds inherit the functional properties of their template, suggesting that transferable pathogenic and non-pathogenic states likely influence the initial engagement between exogenous α-Syn seeds and endogenous neuronal α-Syn. Thus, transmission of synucleinopathies is regulated by biological processes in addition to molecular compatibility.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
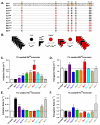
Figure 1. Sequence differences between Huwt and Mswt α-Syn reduce cross-seeded fibrillization rate in vitro
(A), Amino-acid sequences of Huwt and Mswt α-Syn are denoted in red and black, respectively. Divergent residues are highlighted. Chimeric α-Syn proteins contain orthologous residues at the indicated positions. (B), Schematic of the proposed molecular mechanism for cross-seeded α-Syn aggregation. Huwt monomer (black circles) readily add to Huwt PFFs (black wedges) through _cis_-templating, but recruitment of Mswt monomer (red circles) is slower due to imperfect _trans_-templating. Upon overcoming this initial step, recruitment of Mswt monomer is facilitated by complete sequence homology to hybrid PFFs. (C,E), Normalized initiation rates for 1% PFF seeded Mswt (C) and Huwt (E) monomer. Data represent time to reach 50% aggregation. (D,F), Normalized elongation rates (slope from 10%-90% pelletable fibrils) for 1% PFF seeded Mswt (D) and Huwt (F). All data shown as mean ± SEM (_N_=4). Dashed lines denote rate for unseeded reactions. One-way ANOVA (Tukey post-hoc), *p<0.05, **p<0.01, *** p<0.001 vs Huwt PFFs. See also Figures S1-S3.
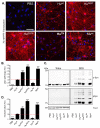
Figure 2. Sequence-dependent and -independent impediments to α-Syn seeding in neurons
(A) Immunostaining of pSyn (red) in primary hippocampal neurons treated at DIV10 for 7d with 70 nM of indicated α-Syn PFFs. Nuclei are stained with DAPI (blue). Scale bar = 50 μm. (B) Quantification of pSyn immunoreactivity in cultures [(area occupied × density)/DAPI count]. Results shown as mean ± SEM (_N_=4) relative to Huwt levels of pSyn (dashed line). (C) Primary hippocampal neurons treated with PFFs were sequentially extracted with 1% Triton X-100 and 2% SDS. Lysates were immunoblotted for total α-Syn or, pSyn, with βIII tubulin (TUJ1) as a loading control. (D) Densitometric quantification of insoluble α-Syn fractions in experiment in C. Results shown as mean ± SEM (N=3). *p < 0.05; **p < 0.01, *** p<0.001, one-way ANOVA vs Huwt PFF treatment (Tukey post-hoc test). See also Figure S4.
Figure 3. Cross-seeding efficiencyof Huwt and Mswt α-Syn PFFs in vivo
Wt mice were injected with either wt (Hu or Ms) or chimeric (HuS87N, HuTN, or HuTNG) α-Syn PFFs via a single unilateral injection into the dorsal striatum. IHC for pSyn was used to visualize LB- and LN-like inclusions. Representative images are shown for piriform cortex (Pir), frontal agranular cortex (FC) or olfactory mitral cell layer (MCL) ipsilateral to the injection site at 30 dpi. Inclusions are visible by 30 dpi in all three regions following treatment with Mswt, HuS87N, or HuTNG PFFs whereas Huwt induced mild α-Syn accumulation in processes (arrowheads). No pathology was detectable in animals that received HuTN PFFs. Scale bars = 30 μm. *p < 0.05; **p < 0.01, one-way ANOVA vs Mswt PFF treatment (Tukey post-hoc test).
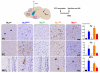
Figure 4. Relative pathological seeding efficacy of wt and chimeric α-Syn PFFs is preserved in vivo
(A) Wt mice were sacrificed at 14, 30, or 90 d after intrastriatal injection of wt or chimeric α-Syn PFFs. (B) Inclusion pathology in the ipsilateral amygdala revealed by anti-pSyn, Syn506, or thioflavin S (ThS). Injected α-Syn PFFs are arranged with increasingly sequence homology to the host Mswt α-Syn from top to bottom. Pathology is detectable in mice injected with Mswt, HuS87N, and HuTNG PFFs at 14 dpi. HuA53T PFF injected mice developed sparse pSyn immunoreactive processes (inset) but no amygdala pathology was detectable in the Huwt cohort until 30 dpi. Scale bars = 50 μm. (C) Quantification of α-Syn pathology at each time point. Data expressed as mean % area occupied by pSyn inclusions ± SD (N ≥4 per group).
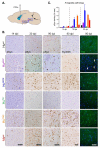
Figure 5. α-Syn pathology in midbrain DA neurons following injections of different α-Syn PFFs
(A) Representative images of pSyn immunoreactive inclusions accumulating in SNpc DA neurons at 30 or 90d after inoculation with α-Syn PFFs. PFFs are listed in order of increasing homology to Mswt α-Syn. Inclusions were detectable in all cohorts except HuTN PFFs which remained clear of pSyn accumulation up to 90 dpi (white arrowheads). Scale bars = 100 μm. (B) Quantification of the proportion of SNpc DA neurons containing pSyn-positive inclusions. Initiation of α-Syn pathology is significantly delayed in mice treated with Huwt PFFs compared to Ms and chimeric PFFs. (C) DA neuron number in the SNpc after treatment with indicated wt or chimeric PFFs at 30, 90, and 180 dpi. All data shown are for the hemisphere ipsilateral to the injection site and shown as mean ± SEM (N = 5-8 per group). No inclusions were detected in the contralateral SNpc and neuron numbers there were unchanged for all groups. (D) Rotarod performance of α-Syn PFF-injected mice at 30, 90, and 180 dpi with indicated PFFs. *p < 0.05, **p <0.01, ***p < 0.001, two-way ANOVA (Tukey’s HSD). See also Figure S5.
Figure 6. Protease treatment of wt and chimeric PFFs reveal distinct digestion patterns
Wt and chimeric α-Syn PFFs were digested with Proteinase K (A) or Pronase E (B) at the indicated concentrations. Digestion products were resolved on a 12% Bis-tris gel and visualized with Coomassie Brilliant Blue. Major bands generated by each enzyme are indicated in red. (C-G) Quantification of specific PFF digestion fragments are shown. Results represent mean band intensity relative to undigested PFFs. Data shown as mean ± SEM from ≥3 independent digestion experiments. *p < 0.05, **p <0.01, ***p < 0.001, two-way ANOVA (Bonferroni test) for all groups vs. HuTN, HuTNG, and Mswt (C); Mswt vs. all groups (D); Huwt vs. all groups (E); HuTNG vs. all groups (F); HuTN vs. all groups (G). See also Figures S5-S7.
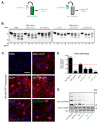
Figure 7. Pathogenic and non-pathogenic properties of PFFs are transferable
(A) Schematic illustrating generation of hybrid fibrils through seeding with pathogenic and non-pathogenic PFFs. (B) Wt and chimeric α-Syn PFFs assembled de novo were digested with PK at the indicated concentrations. Digestion products in Bis-tris gels were stained with Coomassie Brilliant Blue. (C) Immunostaining for pSyn in primary hippocampal neurons treated with Huwt PFFs (140 nM or 28 nM to test the effect of the quantity used for seeding), HuTN[Huwt] PFFs (20% HuWT seeded HuTN PFFs), HuTN PFFs, and Huwt[HuTN] PFFs (20%HuTN seeded Huwt PFFs) at 140 nM. Primary hippocampal neurons (DIV10) were treated with α-Syn PFFs and 7d later cells were fixed and stained. Nuclei are stained with DAPI (blue). Scale bar = 50 μM. (D) Quantification of pSyn levels in PFF-treated neurons [(area occupied × average intensity)/DAPI]. Results shown as mean ± SEM (_N_=3; *p < 0.05; **p < 0.01, one-way ANOVA vs Huwt PFF treatment with Tukey post-hoc test). (E) DIV10 primary hippocampal neurons were treated for 7d with 140 nM PFFs and then sequentially extracted with 1% Triton X-100 followed by 2% SDS. Lysates from Triton and SDS fractions were immunoblotted with Syn9027 (total α-Syn), MJF-R13 (pSyn), and TUJ1 loading control. See also Figure S7.
Similar articles
- Selective imaging of internalized proteopathic α-synuclein seeds in primary neurons reveals mechanistic insight into transmission of synucleinopathies.
Karpowicz RJ Jr, Haney CM, Mihaila TS, Sandler RM, Petersson EJ, Lee VM. Karpowicz RJ Jr, et al. J Biol Chem. 2017 Aug 11;292(32):13482-13497. doi: 10.1074/jbc.M117.780296. Epub 2017 Jun 13. J Biol Chem. 2017. PMID: 28611062 Free PMC article. - Neurons with Cat's Eyes: A Synthetic Strain of α-Synuclein Fibrils Seeding Neuronal Intranuclear Inclusions.
De Giorgi F, Abdul-Shukkoor MB, Kashyrina M, Largitte LA, De Nuccio F, Kauffmann B, Lends A, Laferrière F, Bonhommeau S, Lofrumento DD, Bousset L, Bezard E, Buffeteau T, Loquet A, Ichas F. De Giorgi F, et al. Biomolecules. 2022 Mar 11;12(3):436. doi: 10.3390/biom12030436. Biomolecules. 2022. PMID: 35327628 Free PMC article. - Sequence- and seed-structure-dependent polymorphic fibrils of alpha-synuclein.
Tanaka G, Yamanaka T, Furukawa Y, Kajimura N, Mitsuoka K, Nukina N. Tanaka G, et al. Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1410-1420. doi: 10.1016/j.bbadis.2019.02.013. Epub 2019 Feb 18. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30790619 - Review: Spreading the word: precise animal models and validated methods are vital when evaluating prion-like behaviour of alpha-synuclein.
Rey NL, George S, Brundin P. Rey NL, et al. Neuropathol Appl Neurobiol. 2016 Feb;42(1):51-76. doi: 10.1111/nan.12299. Neuropathol Appl Neurobiol. 2016. PMID: 26666838 Review. - Dissecting the potential molecular mechanisms underlying alpha-synuclein cell-to-cell transfer in Parkinson's disease.
Angot E, Brundin P. Angot E, et al. Parkinsonism Relat Disord. 2009 Dec;15 Suppl 3:S143-7. doi: 10.1016/S1353-8020(09)70802-8. Parkinsonism Relat Disord. 2009. PMID: 20082977 Review.
Cited by
- Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential.
Ferreira N, Gram H, Sorrentino ZA, Gregersen E, Schmidt SI, Reimer L, Betzer C, Perez-Gozalbo C, Beltoja M, Nagaraj M, Wang J, Nowak JS, Dong M, Willén K, Cholak E, Bjerregaard-Andersen K, Mendez N, Rabadia P, Shahnawaz M, Soto C, Otzen DE, Akbey Ü, Meyer M, Giasson BI, Romero-Ramos M, Jensen PH. Ferreira N, et al. Acta Neuropathol. 2021 Jul;142(1):87-115. doi: 10.1007/s00401-021-02316-0. Epub 2021 May 12. Acta Neuropathol. 2021. PMID: 33978813 Free PMC article. - Dopamine neurons exhibit emergent glutamatergic identity in Parkinson's disease.
Steinkellner T, Conrad WS, Kovacs I, Rissman RA, Lee EB, Trojanowski JQ, Freyberg Z, Roy S, Luk KC, Lee VM, Hnasko TS. Steinkellner T, et al. Brain. 2022 Apr 29;145(3):879-886. doi: 10.1093/brain/awab373. Brain. 2022. PMID: 35258081 Free PMC article. - Carboxy-terminal truncations of mouse α-synuclein alter aggregation and prion-like seeding.
Sorrentino ZA, Xia Y, Gorion KM, Hass E, Giasson BI. Sorrentino ZA, et al. FEBS Lett. 2020 Apr;594(8):1271-1283. doi: 10.1002/1873-3468.13728. Epub 2020 Jan 24. FEBS Lett. 2020. PMID: 31912891 Free PMC article. - Rapid Induction of Dopaminergic Neuron Loss Accompanied by Lewy Body-Like Inclusions in A53T BAC-SNCA Transgenic Mice.
Okuda S, Uemura N, Sawamura M, Taguchi T, Ikuno M, Uemura MT, Yamakado H, Takahashi R. Okuda S, et al. Neurotherapeutics. 2022 Jan;19(1):289-304. doi: 10.1007/s13311-021-01169-5. Epub 2021 Dec 21. Neurotherapeutics. 2022. PMID: 34935120 Free PMC article. - Alpha-synuclein oligomers: a new hope.
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Bengoa-Vergniory N, et al. Acta Neuropathol. 2017 Dec;134(6):819-838. doi: 10.1007/s00401-017-1755-1. Epub 2017 Aug 12. Acta Neuropathol. 2017. PMID: 28803412 Free PMC article. Review.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1430–1435. - PMC - PubMed
- Biere AL, Wood SJ, Wypych J, Steavenson S, Jiang Y, Anafi D, Jacobsen FW, Jarosinski MA, Wu GM, Louis JC, et al. Parkinson's disease-associated alpha-synuclein is more fibrillogenic than beta- and gamma-synuclein and cannot cross-seed its homologs. The Journal of biological chemistry. 2000;275:34574–34579. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous