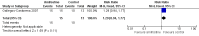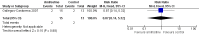Medical interventions for the prevention of platinum-induced hearing loss in children with cancer - PubMed (original) (raw)
Review
Medical interventions for the prevention of platinum-induced hearing loss in children with cancer
Jorrit W van As et al. Cochrane Database Syst Rev. 2016.
Update in
- Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2019 May 7;5(5):CD009219. doi: 10.1002/14651858.CD009219.pub5. Cochrane Database Syst Rev. 2019. PMID: 31063591 Free PMC article.
Abstract
Background: Platinum-based therapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used to treat a variety of paediatric malignancies. One of the most important adverse effects is the occurrence of hearing loss or ototoxicity. In an effort to prevent this ototoxicity, different otoprotective medical interventions have been studied. This review is the second update of a previously published Cochrane review.
Objectives: To assess the efficacy of medical interventions to prevent hearing loss and to determine possible effects of these interventions on anti-tumour efficacy, toxicities other than hearing loss and quality of life in children with cancer treated with platinum-based therapy.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 6), MEDLINE (PubMed) (1945 to 8 July 2016) and EMBASE (Ovid) (1980 to 8 July 2016). In addition, we handsearched reference lists of relevant articles and we assessed the conference proceedings of the International Society for Paediatric Oncology (2006 up to and including 2015), the American Society of Pediatric Hematology/Oncology (2007 up to and including 2016) and the International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer (2010 up to and including 2015). We scanned the International Standard Randomized Controlled Trial Number (ISRCTN) Register (www.isrctn.com) and the National Institute of Health Register (www.clinicaltrials.gov) for ongoing trials (both searched on 12 July 2016).
Selection criteria: Randomized controlled trials (RCTs) or controlled clinical trials (CCTs) evaluating platinum-based therapy together with an otoprotective medical intervention versus platinum-based therapy with placebo, no additional treatment or another protective medical intervention in children with cancer.
Data collection and analysis: Two review authors independently performed the study selection, data extraction, risk of bias assessment and GRADE assessment of included studies, including adverse effects. We performed analyses according to the Cochrane Handbook for Systematic Reviews of Interventions.
Main results: We identified two RCTs and one CCT (total number of participants 149) evaluating the use of amifostine versus no additional treatment in the original version of the review; the updates identified no additional studies. Two studies included children with osteosarcoma, and the other study included children with hepatoblastoma. Children received cisplatin only or a combination of cisplatin and carboplatin, either intra-arterially or intravenously. Pooling of results of the included studies was not possible. However, in the individual studies there was no significant difference in symptomatic ototoxicity only (that is, grade 2 or higher) and combined asymptomatic and symptomatic ototoxicity (that is, grade 1 or higher) between children treated with or without amifostine. Only one study, including children with osteosarcoma treated with intra-arterial cisplatin, provided information on tumour response, defined as the number of participants with a good or partial remission. The available data analysis (data were missing for one participant), best case scenario analysis and worst case scenario analysis all showed a difference in favour of amifostine, but this difference was significant only in the worst case scenario analysis (P = 0.04). There was no information on survival for any of the included studies. Only one study, including children with osteosarcoma treated with intra-arterial cisplatin, provided data on the number of participants with adverse effects other than ototoxicity grade 3 or higher. There was a significant difference in favour of the control group in the occurrence of vomiting grade 3 or 4 (risk ratio (RR) 9.04; 95% confidence interval (CI) 1.99 to 41.12; P = 0.004). There was no significant difference between treatment groups for cardiotoxicity and renal toxicity grade 3 or 4. None of the studies evaluated quality of life. The quality of evidence for the different outcomes was low. We found no eligible studies for possible otoprotective medical interventions other than amifostine and other types of malignancies.
Authors' conclusions: At the moment there is no evidence from individual studies in children with osteosarcoma or hepatoblastoma treated with different platinum analogues and dosage schedules that underscores the use of amifostine as an otoprotective intervention as compared to no additional treatment. Since pooling of results was not possible and all studies had serious methodological limitations, no definitive conclusions can be made. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. Based on the currently available evidence, we are unable to give recommendations for clinical practice. We identified no eligible studies for other possible otoprotective medical interventions and other types of malignancies, so no conclusions can be made about their efficacy in preventing ototoxicity in children treated with platinum-based therapy. More high quality research is needed.
Conflict of interest statement
None known.
Figures
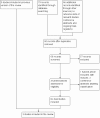
Figure 1
Flow diagram of selection of studies.
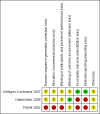
Figure 2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 3
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.1 Ototoxicity according to NCI CTC v2 criteria with intra‐arterial platinum (combined asymptomatic and symptomatic disease).
Figure 4
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.4 Ototoxicity according to NCI CTC v2 criteria with intra‐arterial platinum (symptomatic disease).
Figure 5
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.2 Ototoxicity according to NCI CTC v2 criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
Figure 6
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.5 Ototoxicity according to NCI CTC v2 criteria with intravenous platinum (symptomatic disease).
Figure 7
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.3 Ototoxicity according to modified Brock criteria (combined asymptomatic and symptomatic disease).
Figure 8
Forest plot of comparison: 1 Amifostine versus no otoprotective intervention, outcome: 1.6 Ototoxicity according to modified Brock criteria (symptomatic disease).
Analysis 1.1
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 1 Ototoxicity according to NCI CTC v2 criteria with intra‐arterial platinum (combined asymptomatic and symptomatic disease).
Analysis 1.2
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 2 Ototoxicity according to NCI CTC v2 criteria with intravenous platinum (combined asymptomatic and symptomatic disease).
Analysis 1.3
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 3 Ototoxicity according to modified Brock criteria (combined asymptomatic and symptomatic disease).
Analysis 1.4
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 4 Ototoxicity according to NCI CTC v2 criteria with intra‐arterial platinum (symptomatic disease).
Analysis 1.5
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 5 Ototoxicity according to NCI CTC v2 criteria with intravenous platinum (symptomatic disease).
Analysis 1.6
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 6 Ototoxicity according to modified Brock criteria (symptomatic disease).
Analysis 1.7
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 7 Tumour response (good remission and partial remission).
Analysis 1.8
Comparison 1 Amifostine versus no otoprotective intervention, Outcome 8 Adverse effects other than ototoxicity (vomiting ≥ grade 3).
Similar articles
- Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2018 Jul 5;7(7):CD010885. doi: 10.1002/14651858.CD010885.pub4. Cochrane Database Syst Rev. 2018. PMID: 29975402 Free PMC article. Updated. Review. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD009219. doi: 10.1002/14651858.CD009219.pub3. Cochrane Database Syst Rev. 2014. PMID: 24984156 Review. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2019 May 7;5(5):CD009219. doi: 10.1002/14651858.CD009219.pub5. Cochrane Database Syst Rev. 2019. PMID: 31063591 Free PMC article. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2012 May 16;(5):CD009219. doi: 10.1002/14651858.CD009219.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592737 Updated. Review. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2016 Aug 8;(8):CD010885. doi: 10.1002/14651858.CD010885.pub3. Cochrane Database Syst Rev. 2016. PMID: 27498707 Updated. Review.
Cited by
- Auditory complications among childhood cancer survivors and health-related quality of life: a PanCareLIFE study.
Strebel S, Baust K, Grabow D, Byrne J, Langer T, Am Zehnhoff-Dinnesen A, Kuonen R, Weiss A, Kepak T, Kruseova J, Berger C, Calaminus G, Sommer G, Kuehni CE; PanCareLIFE Consortium. Strebel S, et al. J Cancer Surviv. 2023 Sep 22. doi: 10.1007/s11764-023-01456-4. Online ahead of print. J Cancer Surviv. 2023. PMID: 37736773 - Looking beyond the audiogram in ototoxicity associated with platinum-based chemotherapy.
Baguley DM, Prayuenyong P. Baguley DM, et al. Cancer Chemother Pharmacol. 2020 Feb;85(2):245-250. doi: 10.1007/s00280-019-04012-z. Epub 2019 Dec 21. Cancer Chemother Pharmacol. 2020. PMID: 31865419 Free PMC article. Review. - Sphingomyelin synthase 2 overexpression promotes cisplatin-induced apoptosis of HepG2 cells.
Luo S, Pan Z, Liu S, Yuan S, Yan N. Luo S, et al. Oncol Lett. 2018 Jan;15(1):483-488. doi: 10.3892/ol.2017.7309. Epub 2017 Oct 31. Oncol Lett. 2018. PMID: 29375716 Free PMC article. - Prevention of cisplatin-induced hearing loss in children: Informing the design of future clinical trials.
Minasian LM, Frazier AL, Sung L, O'Mara A, Kelaghan J, Chang KW, Krailo M, Pollock BH, Reaman G, Freyer DR. Minasian LM, et al. Cancer Med. 2018 Jul;7(7):2951-2959. doi: 10.1002/cam4.1563. Epub 2018 May 30. Cancer Med. 2018. PMID: 29846043 Free PMC article. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2018 Jul 5;7(7):CD010885. doi: 10.1002/14651858.CD010885.pub4. Cochrane Database Syst Rev. 2018. PMID: 29975402 Free PMC article. Updated. Review.
References
References to studies included in this review
- Gallegos‐Castorena S, Martínez‐Avalos A, Mohar‐Betancourt A, Guerrero‐Avendaño G, Zapata‐Tarrés M, Medina‐Sansón A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatric Hematology and Oncology 2007;24(6):403‐8. - PubMed
- Katzenstein HM, Chang KW, Krailo M, Chen Z, Finegold MJ, Rowland J, et al. Amifostine does not prevent platinum‐induced hearing loss associated with the treatment of children with hepatoblastoma: a report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group. Cancer 2009;115(24):5828‐35. - PMC - PubMed
- Petrilli AS, Oliveira DT, Ginani VC, Kechichian R, Dishtchekenian A, Medeiros Roque Filho W, et al. Use of amifostine in the therapy of osteosarcoma in children and adolescents. Journal of Pediatric Hematology/Oncology 2002;24(3):188‐91. - PubMed
- Petrilli AS, Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus‐Garcia R, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. Journal of Clinical Oncology 2006;24(7):1161‐8. - PubMed
References to studies excluded from this review
- Doolittle ND, Muldoon LL, Brummett RE, Tyson RM, Lacy C, Bubalo JS, et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin‐induced hearing loss in patients with malignant brain tumors. Clinical Cancer Research 2001;7(3):493‐500. - PubMed
- Elsendoorn TJ, Weijl NI, Mithoe S, Zwinderman AH, Dam F, Zwart FA, et al. Chemotherapy‐induced chromosomal damage in peripheral blood lymphocytes of cancer patients supplemented with antioxidants or placebo. Mutation Research 2001;498(1‐2):145‐58. - PubMed
References to studies awaiting assessment
- Freyer DR. The effects of sodium thiosulfate (STS) on cisplatin‐induced hearing loss: a report from the Children's Oncology Group. Journal of Clinical Oncology 2014;5s:abstract 10017.
- Maibach R, Childs M, Rajput K, Neuwelt EA, Roebuck D, Sullivan MJ, et al. SIOPEL 6: a multicenter open‐label randomized phase III trial of the efficacy of sodium thiosulphate (STS) in reducing ototoxicity in patients receiving cisplatin (Cis) monotherapy for standard‐risk hepatoblastoma (SR‐HB). Journal of Clinical Oncology 2014;5s:abstract TPS10094.
Additional references
- Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound‐related ototoxicity in children: long‐term follow‐up reveals continuous worsening of hearing loss. Journal of Pediatric Hematology/Oncology 2004;26(10):649‐55. - PubMed
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Medical and Pediatric Oncology 1991;19(4):295‐300. - PubMed
- Jenney MEM. Late effects of cancer treatment and current protective measures. In: Voûte PA, Barett A, Stevens MCG, Caron HN editor(s). Cancer in Children. Fifth. Oxford University Press, 2005:123‐37.
- Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin‐containing regimens. Journal of Pediatric Hematology/Oncology 2008;30(2):130‐4. - PubMed
- Eloxatin summary of product characteristics. www.sanofi‐aventis.co.uk/products/Eloxatin_SPC.pdf (assessed 2 March 2010).
References to other published versions of this review
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources