Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer - PubMed (original) (raw)
Evaluation of antibody level against Fusobacterium nucleatum in the serological diagnosis of colorectal cancer
Hai-Fang Wang et al. Sci Rep. 2016.
Abstract
Fusobacterium nucleatum (F. nucleatum, Fn) is associated with the colorectal cancer (CRC). Fn-infection could induce significant levels of serum Fn-specific antibodies in human and mice. The objective of this study was to identify Fn-infection that elicit a humoral response in patients with CRC and evaluate the diagnostic performance of serum anti-Fn antibodies. In this work, we showed the mean absorbance value of anti-Fn-IgA and -IgG in the CRC group were significantly higher than those in the benign colon disease group and healthy control group (P < 0.001). The sensitivity and specificity of ELISA for the detection of anti-Fn-IgA were 36.43% and 92.71% based on the optimal cut-off. The combination of anti-Fn-IgA and carcino-embryonic antigen (CEA) was better for diagnosing CRC (Sen: 53.10%, Spe: 96.41%; AUC = 0.848). Furthermore, combining anti-Fn-IgA with CEA and carbohydrate antigen 19-9 (CA19-9) (Sen: 40.00%, Spe: 94.22%; AUC = 0.743) had the better ability to classify CRC patients with stages I-II. These results suggested that Fn-infection elicited high level of serum anti-Fn antibodies in CRC patients, and serum anti-Fn-IgA level may be a potential diagnosing biomarker for CRC. Serum anti-Fn-IgA in combination with CEA and CA19-9 increases the sensitivity of detecting early CRC.
Figures
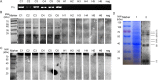
Figure 1. Detection of the specific antigen of Fn that leads to an intense immune response of CRC patients.
(A) PCR was detected by PCR in stool of 6 CRC patients and healthy controls respectively (C: CRC patients; H: healthy controls; neg: negative control). (B,C) Antigens reactive with anti-Fn-IgA (B) and anti-Fn-IgG (C) were determined by western blotting through incubating with a reference serum dilution of 6 Fn-positive CRC patients or 6 Fn-negative healthy individual as primary antibody. (D) The whole proteins of Fn were separated by 10% SDS-PAGE and then stained with Coomassie brilliant blue (lane 1) and the specific antigens that caused high levels of anti-Fn-IgA were detected by western blotting through incubating with mixed serum samples of 6 CRC patients as primary antibody (lane 2). Of note, Fig. 1B–D were cropped from a single image on the dashed lines to be better presented in the article’s context. The gels have been run under the same conditions and subsequently processed with the same set of materials. These three complete figures could be found respectively in the Supplementary Figs S1 and S2.
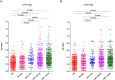
Figure 2. Comparison of OD values of anti-Fn-IgA or anti-Fn-IgG in sera from healthy adult human subjects (HS: healthy subjects, n = 200), benign colon disease (n = 150), stage I-II of CRC (n = 55), stage III-IV of CRC (n = 203), the total of CRC patients (n = 258) were individually assayed.
Symbols indicate individual OD value; horizontal lines indicate mean values ± SD. Differences between the five groups were analyzed by Kruskal-Wallis test. (A) anti-Fn-IgA. (B) anti-Fn-IgG.
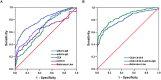
Figure 3. Diagnostic outcomes for serum anti-Fn-IgA, anti-Fn-IgG, CEA or CA19-9 alone or in combination in the diagnosis of CRC.
(A) ROC curves for the diagnostic strength to identify CRC using anti-Fn-IgA, anti-Fn-IgG, CEA or CA19-9 level. (anti-Fn-IgA: AUC = 0.704; anti-Fn-IgG: AUC = 0.645; CEA: AUC = 0.796; CA19-9: AUC = 0.635). (B) ROC curves for the diagnostic strength to identify CRC using anti-Fn-IgA, CEA or CA19-9. (CEA+ CA19-9: AUC = 0.809; anti-Fn-IgA + CEA+ CA19-9: AUC = 0.858).
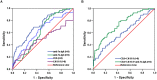
Figure 4. Diagnostic outcomes for serum anti-Fn-IgA, anti-Fn-IgG, CEA or CA19-9 alone or in combination in the diagnosis of early stage CRC.
(A) ROC curves for the diagnostic strength to identify early CRC using anti-Fn-IgA, anti-Fn-IgG, CEA or CA19-9 level. (anti-Fn-IgA: AUC = 0.709 anti-Fn-IgG: AUC = 0.612; CEA: AUC = 0.624; CA19-9: AUC = 0.492). (B) ROC curves for the diagnostic strength to identify CRC using anti-Fn-IgA, CEA or CA19-9. (CEA+ CA19-9: AUC = 0.605; anti-Fn-IgA + CEA+ CA19-9: AUC = 0.743).
Similar articles
- Improved diagnosis of colorectal cancer using combined biomarkers including Fusobacterium nucleatum, fecal occult blood, transferrin, CEA, CA19-9, gender, and age.
Zhao R, Xia D, Chen Y, Kai Z, Ruan F, Xia C, Gong J, Wu J, Wang X. Zhao R, et al. Cancer Med. 2023 Jul;12(13):14636-14645. doi: 10.1002/cam4.6067. Epub 2023 May 10. Cancer Med. 2023. PMID: 37162269 Free PMC article. - Circulating IgA Antibodies Against Fusobacterium nucleatum Amyloid Adhesin FadA are a Potential Biomarker for Colorectal Neoplasia.
Baik JE, Li L, Shah MA, Freedberg DE, Jin Z, Wang TC, Han YW. Baik JE, et al. Cancer Res Commun. 2022 Nov 29;2(11):1497-1503. doi: 10.1158/2767-9764.CRC-22-0248. eCollection 2022 Nov. Cancer Res Commun. 2022. PMID: 36970057 Free PMC article. - Diagnostic accuracy of Fusobacterium nucleatum IgA and IgG ELISA test in colorectal cancer.
Kurt M, Yumuk Z. Kurt M, et al. Sci Rep. 2021 Jan 15;11(1):1608. doi: 10.1038/s41598-021-81171-1. Sci Rep. 2021. PMID: 33452405 Free PMC article. - _Fusobacterium nucleatum_-positive colorectal cancer.
Yang Z, Ji G. Yang Z, et al. Oncol Lett. 2019 Aug;18(2):975-982. doi: 10.3892/ol.2019.10433. Epub 2019 Jun 4. Oncol Lett. 2019. PMID: 31423156 Free PMC article. Review. - Association between Fusobacterium nucleatum and colorectal cancer: Progress and future directions.
Zhang S, Cai S, Ma Y. Zhang S, et al. J Cancer. 2018 Apr 18;9(9):1652-1659. doi: 10.7150/jca.24048. eCollection 2018. J Cancer. 2018. PMID: 29760804 Free PMC article. Review.
Cited by
- Fusobacterium nucleatum Causes Microbial Dysbiosis and Exacerbates Visceral Hypersensitivity in a Colonization-Independent Manner.
Gu X, Song LJ, Li LX, Liu T, Zhang MM, Li Z, Wang P, Li M, Zuo XL. Gu X, et al. Front Microbiol. 2020 Jun 24;11:1281. doi: 10.3389/fmicb.2020.01281. eCollection 2020. Front Microbiol. 2020. PMID: 32733392 Free PMC article. - Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers.
Chen Y, Lu Y, Ke Y, Li Y. Chen Y, et al. Medicine (Baltimore). 2019 Sep;98(39):e17221. doi: 10.1097/MD.0000000000017221. Medicine (Baltimore). 2019. PMID: 31574832 Free PMC article. - Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer.
Raza A, Khan AQ, Inchakalody VP, Mestiri S, Yoosuf ZSKM, Bedhiafi T, El-Ella DMA, Taib N, Hydrose S, Akbar S, Fernandes Q, Al-Zaidan L, Krishnankutty R, Merhi M, Uddin S, Dermime S. Raza A, et al. J Exp Clin Cancer Res. 2022 Mar 15;41(1):99. doi: 10.1186/s13046-022-02318-0. J Exp Clin Cancer Res. 2022. PMID: 35292091 Free PMC article. Review. - Colorectal cancer and gut microbiota studies in China.
Wang Z, Dan W, Zhang N, Fang J, Yang Y. Wang Z, et al. Gut Microbes. 2023 Jan-Dec;15(1):2236364. doi: 10.1080/19490976.2023.2236364. Gut Microbes. 2023. PMID: 37482657 Free PMC article. Review. - Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated With Cystic Pancreatic Neoplasm Malignancy.
Alkharaan H, Lu L, Gabarrini G, Halimi A, Ateeb Z, Sobkowiak MJ, Davanian H, Fernández Moro C, Jansson L, Del Chiaro M, Özenci V, Sällberg Chen M. Alkharaan H, et al. Front Immunol. 2020 Aug 28;11:2003. doi: 10.3389/fimmu.2020.02003. eCollection 2020. Front Immunol. 2020. PMID: 32983143 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous