Antimicrobial Activity and Mechanism of Inhibition of Silver Nanoparticles against Extreme Halophilic Archaea - PubMed (original) (raw)
Antimicrobial Activity and Mechanism of Inhibition of Silver Nanoparticles against Extreme Halophilic Archaea
Rebecca S Thombre et al. Front Microbiol. 2016.
Abstract
Haloarchaea are salt-loving halophilic microorganisms that inhabit marine environments, sea water, salterns, and lakes. The resistance of haloarchaea to physical extremities that challenge organismic survival is ubiquitous. Metal and antibiotic resistance of haloarchaea has been on an upsurge due to the exposure of these organisms to metal sinks and drug resistance genes augmented in their natural habitats due to anthropogenic activities and environmental pollution. The efficacy of silver nanoparticles (SNPs) as a potent and broad spectrum inhibitory agent is known, however, there are no reports on the inhibitory activity of SNPs against haloarchaea. In the present study, we have investigated the antimicrobial potentials of SNPs synthesized using aqueous leaf extract of Cinnamomum tamala against antibiotic resistant haloarchaeal isolates Haloferax prahovense RR8, Haloferax lucentense RR15, Haloarcula argentinensis RR10 and Haloarcula tradensis RR13. The synthesized SNPs were characterized by UV-Vis spectroscopy, scanning electron microscopy, energy dispersive X-ray spectroscopy, dynamic light scattering, X-ray diffraction and Fourier transform infrared spectroscopy. The SNPs demonstrated potent antimicrobial activity against the haloarchaea with a minimum inhibitory concentration of 300-400 μg/ml. Growth kinetics of haloarchaea in the presence of SNPs was studied by employing the Baranyi mathematical model for microbial growth using the DMFit curve fitting program. The C. tamala SNPs also demonstrated cytotoxic activity against human lung adenocarcinoma epithelial cell line (A540) and human breast adenocarcinoma cell line (MCF-7). The mechanism of inhibition of haloarchaea by the SNPs was investigated. The plausible mechanism proposed is the alterations and disruption of haloarchaeal membrane permeability by turbulence, inhibition of respiratory dehydrogenases and lipid peroxidation causing cellular and DNA damage resulting in cell death.
Keywords: Baranyi model; Cinnamomum tamala; antibiotic resistant; antimicrobial; cytotoxicity; extreme haloarchaea; membrane permeability; silver nanoparticles.
Figures
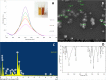
FIGURE 1
(A) UV-Vis spectra of SNP synthesized using C. tamala extract; inset figure: color change from pale yellow (0 h) to dark brown (5 h) indicating synthesis of nanoparticles. (B) FEG-SEM of SNPs synthesized using C. tamala leaf extract depicting spherical nanoparticles. (C) Energy Dispersive X-ray spectrum of SNPs biosynthesized using C. tamala leaf extract. (D) Fourier transform infrared spectra of SNPs synthesized using C. tamala leaf extract.
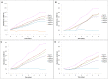
FIGURE 2
Growth curve of Haloarchaea in different concentrations of SNPs: (A)Haloarcula argentinensis, (B)Haloarcula tradensis, (C)Haloferax lucentense, and (D)Haloferax prahovense.
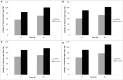
FIGURE 3
Effect of SNPs on membrane leakage of reducing sugars in haloarchaea (A)Haloarcula argentinensis, (B)Haloarcula tradensis, (C)Haloferax lucentense, and (D)Haloferax prahovense. Error bar represents standard error.
FIGURE 4
Effect of SNPs on membrane leakage of proteins in haloarchaea (A)Haloarcula argentinensis, (B)Haloarcula tradensis, (C)Haloferax lucentense, and (D)Haloferax prahovense. Error bar represents standard error.
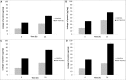
FIGURE 5
Effect of SNPs on respiratory chain dehydrogenase activity in haloarchaea (A)Haloarcula argentinensis, (B)Haloarcula tradensis, (C)Haloferax lucentense, and (D)Haloferax prahovense.
FIGURE 6
Effect of SNPs on membrane lipid peroxidation as detected by MDA content in haloarchaea (A)Haloarcula argentinensis, (B)Haloarcula tradensis, (C)Haloferax lucentense, and (D)Haloferax prahovense. Error bar represents standard error.
FIGURE 7
Proposed mechanism of inhibition of haloarchaea by SNPs. (A) Attachment of SNPs to cell. [1: SNPs, 2: Bacteriorhodopsin protein, 3: Electron transport chain, 4: ATPase complex, 5: Trk type Potassium ion channel, 6: Haloarchaeal genomic DNA, 7: S-layer, 8: Membrane, 9: Intracellular cytosol]. (B) Disruption of membrane integrity and leakage of intracellular constituents. (C) Inhibition of respiratory dehydrogenases and electron transport chain and generation of MDA and ROS. (D) Oxidative, DNA and cellular damage caused by ROS leading to cell death.
Similar articles
- Antimicrobial potentials of Helicteres isora silver nanoparticles against extensively drug-resistant (XDR) clinical isolates of Pseudomonas aeruginosa.
Mapara N, Sharma M, Shriram V, Bharadwaj R, Mohite KC, Kumar V. Mapara N, et al. Appl Microbiol Biotechnol. 2015 Dec;99(24):10655-67. doi: 10.1007/s00253-015-6938-x. Epub 2015 Sep 11. Appl Microbiol Biotechnol. 2015. PMID: 26362684 - Biology and survival of extremely halophilic archaeon Haloarcula marismortui RR12 isolated from Mumbai salterns, India in response to salinity stress.
Thombre RS, Shinde VD, Oke RS, Dhar SK, Shouche YS. Thombre RS, et al. Sci Rep. 2016 May 27;6:25642. doi: 10.1038/srep25642. Sci Rep. 2016. PMID: 27231230 Free PMC article. - Bioactive molecules from haloarchaea: Scope and prospects for industrial and therapeutic applications.
Moopantakath J, Imchen M, Anju VT, Busi S, Dyavaiah M, Martínez-Espinosa RM, Kumavath R. Moopantakath J, et al. Front Microbiol. 2023 Mar 31;14:1113540. doi: 10.3389/fmicb.2023.1113540. eCollection 2023. Front Microbiol. 2023. PMID: 37065149 Free PMC article. Review. - Haloarchaeal Carotenoids: Healthy Novel Compounds from Extreme Environments.
Giani M, Garbayo I, Vílchez C, Martínez-Espinosa RM. Giani M, et al. Mar Drugs. 2019 Sep 6;17(9):524. doi: 10.3390/md17090524. Mar Drugs. 2019. PMID: 31500208 Free PMC article. Review.
Cited by
- Antibacterial activity and mechanism of X33 antimicrobial oligopeptide against Acinetobacter baumannii.
Lu Q, Wu X, Fang Y, Wang Y, Zhang B. Lu Q, et al. Synth Syst Biotechnol. 2024 Mar 15;9(2):312-321. doi: 10.1016/j.synbio.2024.03.002. eCollection 2024 Jun. Synth Syst Biotechnol. 2024. PMID: 38545458 Free PMC article. - A Twist to the Kirby-Bauer Disk Diffusion Susceptibility Test: an Accessible Laboratory Experiment Comparing Haloferax volcanii and Escherichia coli Antibiotic Susceptibility to Highlight the Unique Cell Biology of Archaea.
Schiller H, Young C, Schulze S, Tripepi M, Pohlschroder M. Schiller H, et al. J Microbiol Biol Educ. 2022 Jan 31;23(1):e00234-21. doi: 10.1128/jmbe.00234-21. eCollection 2022 Apr. J Microbiol Biol Educ. 2022. PMID: 35340443 Free PMC article. - Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics.
Krishnamoorthy R, Athinarayanan J, Periyasamy VS, Alshuniaber MA, Alshammari G, Hakeem MJ, Ahmed MA, Alshatwi AA. Krishnamoorthy R, et al. Molecules. 2022 Apr 12;27(8):2489. doi: 10.3390/molecules27082489. Molecules. 2022. PMID: 35458685 Free PMC article. - Bacterioruberin: Biosynthesis, Antioxidant Activity, and Therapeutic Applications in Cancer and Immune Pathologies.
Giani M, Pire C, Martínez-Espinosa RM. Giani M, et al. Mar Drugs. 2024 Apr 9;22(4):167. doi: 10.3390/md22040167. Mar Drugs. 2024. PMID: 38667784 Free PMC article. Review. - Biogenic Silver Nanoparticles Strategically Combined With Origanum vulgare Derivatives: Antibacterial Mechanism of Action and Effect on Multidrug-Resistant Strains.
Scandorieiro S, Rodrigues BCD, Nishio EK, Panagio LA, de Oliveira AG, Durán N, Nakazato G, Kobayashi RKT. Scandorieiro S, et al. Front Microbiol. 2022 May 6;13:842600. doi: 10.3389/fmicb.2022.842600. eCollection 2022. Front Microbiol. 2022. PMID: 35602016 Free PMC article.
References
- Agnihotri M., Joshi S., Kumar A. R., Zinjarde S., Kulkarn S. (2009). Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 63 1231–1234. 10.1016/j.matlet.2009.02.042 - DOI
- Ahmad A., Senapathi S., Khan M., Ramani R. (2003). Intracellular synthesis of gold nanoparticles by a novel alkotolerant actinomycetes, Rhodococcus species. Nanotechnology 14 824–828. 10.1088/0957-4484/14/7/323 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources