Astaxanthin affects oxidative stress and hyposalivation in aging mice - PubMed (original) (raw)
Astaxanthin affects oxidative stress and hyposalivation in aging mice
Manatsu Kuraji et al. J Clin Biochem Nutr. 2016 Sep.
Abstract
Oral dryness, a serious problem for the aging Japanese society, is induced by aging-related hyposalivation and causes dysphagia, dysgeusia, inadaptation of dentures, and growth of oral Candida albicans. Oxidative stress clearly plays a role in decreasing saliva secretion and treatment with antioxidants such astaxanthin supplements may be beneficial. Therefore, we evaluated the effects of astaxanthin on the oral saliva secretory function of aging mice. The saliva flow increased in astaxanthin-treated mice 72 weeks after administration while that of the control decreased by half. The plasma d-ROMs values of the control but not astaxanthin-treated group measured before and 72 weeks after treatment increased. The diacron-reactive oxygen metabolites (d-ROMs) value of astaxanthin-treated mice 72 weeks after treatment was significantly lower than that of the control group was. The plasma biological antioxidative potential (BAP) values of the control but not astaxanthin-treated mice before and 72 weeks after treatment decreased. Moreover, the BAP value of the astaxanthin-treated group 72 weeks after treatment was significantly higher than that of the control was. Furthermore, the submandibular glands of astaxanthin-treated mice had fewer inflammatory cells than the control did. Specifically, immunofluorescence revealed a significantly large aquaporin-5 positive cells in astaxanthin-treated mice. Our results suggest that astaxanthin treatment may prevent age-related decreased saliva secretion.
Keywords: aquaporin-5; astaxanthin; hyposalivation; inflammation; oral dryness.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
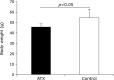
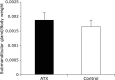
Fig. 1
Comparison of body weight of astaxanthin (AX) and control groups. Body weight of AX-treated group is significantly lower than that of control group 72 weeks after AX treatment (p<0.05).
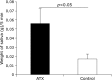
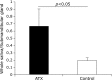
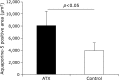
Fig. 2
Comparison of saliva amounts between astaxanthin (AX) and control groups. Saliva flow of AX group was significantly higher than that of control group (p<0.05).
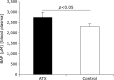
Fig. 3
Comparison of Diacron-reactive oxygen metabolites (D-ROMs) value of astaxanthin (AX) and control groups. D-ROMs value of AX group 72 weeks after AX administration was significantly lower than that of control group (p<0.05).
Fig. 4
Comparison of biological antioxidative potential (BAP) values of astaxanthin (AX) and control groups. BAP value of AX group 72 weeks after AX administration was significantly higher than that of control group.
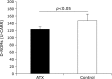
Fig. 5
Changes in ratio of mandibular gland weight to whole body weight. No significant differences were observed in mandibular gland weight/body weight ratio between the astaxanthin (AX) and control groups.
Fig. 6
Changes in ratio of salivary flow to weight of mandibular gland. Ratio of saliva flow and mandibular gland weight was significantly higher in astaxanthin (AX) group than in control group.
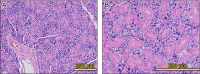
Fig. 7
Hematoxylin and eosin (H&E) staining of astaxanthin (AX) group submandibular glands. Submandibular glands of AX-treated group had several large striated ducts (A). Striated ducts were filled with secretory granules present in acinar cells (B).
Fig. 8
Hematoxylin and eosin (H&E) staining of control group submandibular glands. Inflammatory cells infiltration (A) and atrophic acinar cells (B) were observed. Vacuolar or fatty degeneration (C) and fibrous connective tissue (D) were observed.
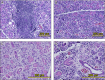
Fig. 9
Immunohistochemically stained image of aquaporin (AQP)-5-positive domain in the mandibular gland (A) Control group. (B) Astaxanthin (AX) group. Mandibular gland of AX group shows numerous AQP-5-positive cells while that of control group shows few.
Fig. 10
Positive area of aquaporin (AQP)-5. Positive domain of AQP-5 in astaxanthin (AX) group covered a larger area than that of control group.
Similar articles
- Relationship between hyposalivation and oxidative stress in aging mice.
Yamauchi Y, Matsuno T, Omata K, Satoh T. Yamauchi Y, et al. J Clin Biochem Nutr. 2017 Jul;61(1):40-46. doi: 10.3164/jcbn.16-79. Epub 2017 Jun 23. J Clin Biochem Nutr. 2017. PMID: 28751808 Free PMC article. - Protective effects of astaxanthin on a combination of D-galactose and jet lag-induced aging model in mice.
Ni Y, Wu T, Yang L, Xu Y, Ota T, Fu Z. Ni Y, et al. Endocr J. 2018 May 28;65(5):569-578. doi: 10.1507/endocrj.EJ17-0500. Epub 2018 Mar 10. Endocr J. 2018. PMID: 29526991 - Inhibitory effects of astaxanthin on azoxymethane-induced colonic preneoplastic lesions in C57/BL/KsJ-db/db mice.
Kochi T, Shimizu M, Sumi T, Kubota M, Shirakami Y, Tanaka T, Moriwaki H. Kochi T, et al. BMC Gastroenterol. 2014 Dec 17;14:212. doi: 10.1186/s12876-014-0212-z. BMC Gastroenterol. 2014. PMID: 25515685 Free PMC article. - Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential.
Kidd P. Kidd P. Altern Med Rev. 2011 Dec;16(4):355-64. Altern Med Rev. 2011. PMID: 22214255 Review. - Retrometabolic syntheses of astaxanthin (3,3'-dihydroxy-beta,beta-carotene-4,4'-dione) conjugates: a novel approach to oral and parenteral cardio-protection.
Lockwood SF, Jackson HL, Gross GJ. Lockwood SF, et al. Cardiovasc Hematol Agents Med Chem. 2006 Oct;4(4):335-49. doi: 10.2174/187152506784111472. Cardiovasc Hematol Agents Med Chem. 2006. PMID: 17073610 Review.
Cited by
- Relationship between hyposalivation and oxidative stress in aging mice.
Yamauchi Y, Matsuno T, Omata K, Satoh T. Yamauchi Y, et al. J Clin Biochem Nutr. 2017 Jul;61(1):40-46. doi: 10.3164/jcbn.16-79. Epub 2017 Jun 23. J Clin Biochem Nutr. 2017. PMID: 28751808 Free PMC article. - Cordycepin attenuates Salivary Hypofunction through the Prevention of Oxidative Stress in Human Submandibular Gland Cells.
Jaiboonma A, Kaokaen P, Chaicharoenaudomrung N, Kunhorm P, Janebodin K, Noisa P, Jitprasertwong P. Jaiboonma A, et al. Int J Med Sci. 2020 Jul 6;17(12):1733-1743. doi: 10.7150/ijms.46707. eCollection 2020. Int J Med Sci. 2020. PMID: 32714076 Free PMC article. - Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells.
Shimokawa T, Yoshida M, Fukuta T, Tanaka T, Inagi T, Kogure K. Shimokawa T, et al. J Clin Biochem Nutr. 2019 Jan;64(1):27-35. doi: 10.3164/jcbn.18-27. Epub 2018 Nov 30. J Clin Biochem Nutr. 2019. PMID: 30705509 Free PMC article. - Astaxanthin ameliorates ferric nitrilotriacetate-induced renal oxidative injury in rats.
Okazaki Y, Okada S, Toyokuni S. Okazaki Y, et al. J Clin Biochem Nutr. 2017 Jul;61(1):18-24. doi: 10.3164/jcbn.16-114. Epub 2017 Jun 15. J Clin Biochem Nutr. 2017. PMID: 28751805 Free PMC article. - Accuracy of a questionnaire on xerostomia as a screening tool for hyposalivation.
de Carvalho HN, Dos Santos YL, Bernardino ÍM, de Lima KC, Granville-Garcia AF, Melo de Brito Costa EM. de Carvalho HN, et al. Int Dent J. 2020 Dec;70(6):427-434. doi: 10.1111/idj.12586. Epub 2020 Aug 23. Int Dent J. 2020. PMID: 32830311 Free PMC article.
References
- Ben-Aryeh H, Miron D, Berdicevsky I, Szargel R, Gutman D. Xerostomia in the elderly: prevalence, diagnosis, complications and treatment. Gerodontology. 1985;4:77–82. - PubMed
- Vissink A, Spijkervet FK, Van Nieuw Amerongen A. Aging and saliva: a review of the literature. Spec Care Dentist. 1996;16:95–103. - PubMed
- Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–543. - PubMed
- Baum BJ. Evaluation of stimulated parotid saliva flow rate in different age groups. J Dent Res. 1981;60:1292–1296. - PubMed
- Parvinen T, Larmas M. Age dependency of stimulated salivary flow rate, pH, and lactobacillus and yeast concentrations. J Dent Res. 1982;61:1052–1055. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources