Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids - PubMed (original) (raw)
Review
. 2016 Nov;280(5):465-475.
doi: 10.1111/joim.12540. Epub 2016 Oct 3.
Affiliations
- PMID: 27699898
- PMCID: PMC5218584
- DOI: 10.1111/joim.12540
Review
Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids
U Smith et al. J Intern Med. 2016 Nov.
Abstract
Obesity, the major cause of the current global epidemic of type 2 diabetes (T2D), induces insulin resistance in peripheral insulin target tissues. Several mechanisms have been identified related to cross-talk between adipose tissue, skeletal muscle and liver. These mechanisms involve both increased free fatty acid release and altered secretion of adipokines from adipose tissue. A major determinant of metabolic health is the ability of subcutaneous adipose tissue (SAT) to store excess fat rather than allowing it to accumulate in ectopic depots including liver (i.e. in nonalcoholic fatty liver disease), muscle and heart, or in epicardial/pericardial and visceral fat depots which promote the metabolic complications of obesity. The ability to recruit and differentiate precursor cells into adipose cells (adipogenesis) in SAT is under genetic regulation and is reduced in high-risk individuals who have first-degree relatives with T2D. Early recruitment of new adipose cells is dependent on the cross-talk between canonical WNT and BMP4 signalling; WNT enhances their undifferentiated and proliferative state whereas BMP4 induces their commitment to the adipogenic lineage. Dysregulation of these signalling pathways is associated with impaired adipogenesis and impaired ability to respond to the need to store excess lipids in SAT. This leads to hypertrophic, dysfunctional and insulin-resistant adipose cells with a reduced content of GLUT4, the major insulin-regulated glucose transporter, which in turn reduces adipose tissue glucose uptake and de novo lipogenesis. We recently identified that reduced GLUT4 and lipogenesis in adipocytes impairs the synthesis of a novel family of lipids secreted by adipose tissue (and potentially other tissues), branched fatty acid esters of hydroxy fatty acids (FAHFAs). FAHFAs have beneficial metabolic effects, including enhancing insulin-stimulated glucose transport and glucose-stimulated GLP1 and insulin secretion, as well as powerful anti-inflammatory effects. FAHFA levels are reduced in subcutaneous adipose tissue in insulin-resistant individuals, and this novel family of lipids may become of future therapeutic use.
Keywords: adipose tissue; glucose; insulin resistance; lipogenesis; type 2 diabetes.
© 2016 The Association for the Publication of the Journal of Internal Medicine.
Conflict of interest statement
statement BBK is an inventor on patents regarding RBP4 and FAHFA lipids.
Figures
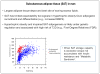
Fig. 1
Adipocyte hypertrophy and associated characteristics. IR, insulin resistant; FDR, first-degree relative., AT, adipose tissue Reproduced from Gustafson et al. (52), with permission.
Fig. 2
Subcutaneous adipose tissue in humans.
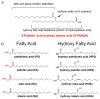
Fig. 3
Structure of branched fatty acid esters of hydroxy fatty acids (FAHFAs). (A) FAHFAs are esters that combine a fatty acid such as palmitate with a hydroxy fatty acid backbone such as hydroxystearic acid. The FAHFA shown is 9-palmitic acid hydroxystearic acid (PAHSA) which is the most upregulated FAHFA family member in adipose tissue of mice that are overexpressing GLUT4 selectively in adipocytes. (B) A total of 16 FAHFA family members were identified in mouse serum. These lipids consist of four different fatty acid moieties and four hydroxy fatty acid moieties in different combinations. Adapted from ref. and reproduced with permission from Cell Press.
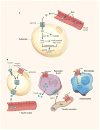
Fig 4
Anti-diabetic and anti-inflammatory effects of branched fatty acid esters of hydroxy fatty acids (FAHFAs). (a) Glucose is transported into adipocytes by the GLUT4 glucose transporter. The increased glucose entry activates the transcription factor ChREBP, thereby enhancing de novo lipogenesis and synthesis of branched Fatty Acid esters of Hydroxy Fatty Acids (FAHFAs). (b) FAHFAs augment insulin-stimulated glucose transport in adipocytes and glucose-stimulated GLP1 secretion from the gut enteroendocrine cells and insulin secretion from pancreatic beta cells. FAHFAs also reduce inflammation by decreasing the production of pro-inflammatory cytokines from macrophages and dendritic cells. ChREBP, carbohydrate response element binding protein; FAS, fatty acid synthase; GPR120, G protein-coupled receptor 120. Diabetes: The good in fat. Muoio D.M & Newgard C.B. Nature 516:49–50, 2014. Reproduced with permission from Nature Publishing Group, license number 3851950681929.
Similar articles
- GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects.
Moraes-Vieira PM, Saghatelian A, Kahn BB. Moraes-Vieira PM, et al. Diabetes. 2016 Jul;65(7):1808-15. doi: 10.2337/db16-0221. Epub 2016 Jun 10. Diabetes. 2016. PMID: 27288004 Free PMC article. Review. - Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity.
Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Hammarstedt A, et al. Physiol Rev. 2018 Oct 1;98(4):1911-1941. doi: 10.1152/physrev.00034.2017. Physiol Rev. 2018. PMID: 30067159 Review. - Energy metabolism of white adipose tissue and insulin resistance in humans.
Bódis K, Roden M. Bódis K, et al. Eur J Clin Invest. 2018 Nov;48(11):e13017. doi: 10.1111/eci.13017. Epub 2018 Sep 26. Eur J Clin Invest. 2018. PMID: 30107041 Review. - Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity.
Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, Hammarstedt A, Hedjazifar S, Hoffmann JM, Nigro C, Mirra P, Fiory F, Formisano P, Miele C, Smith U, Beguinot F. Longo M, et al. Diabetologia. 2018 Feb;61(2):369-380. doi: 10.1007/s00125-017-4471-4. Epub 2017 Oct 24. Diabetologia. 2018. PMID: 29067487 Free PMC article. - Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk.
Gustafson B, Smith U. Gustafson B, et al. Atherosclerosis. 2015 Jul;241(1):27-35. doi: 10.1016/j.atherosclerosis.2015.04.812. Epub 2015 Apr 30. Atherosclerosis. 2015. PMID: 25957567 Review.
Cited by
- The Role of Mondo Family Transcription Factors in Nutrient-Sensing and Obesity.
Ke H, Luan Y, Wu S, Zhu Y, Tong X. Ke H, et al. Front Endocrinol (Lausanne). 2021 Mar 31;12:653972. doi: 10.3389/fendo.2021.653972. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33868181 Free PMC article. Review. - Adipokines as potential biomarkers for type 2 diabetes mellitus in cats.
Sierawska O, Niedźwiedzka-Rystwej P. Sierawska O, et al. Front Immunol. 2022 Sep 30;13:950049. doi: 10.3389/fimmu.2022.950049. eCollection 2022. Front Immunol. 2022. PMID: 36248900 Free PMC article. Review. - Spaceflight alters host-gut microbiota interactions.
Gonzalez E, Lee MD, Tierney BT, Lipieta N, Flores P, Mishra M, Beckett L, Finkelstein A, Mo A, Walton P, Karouia F, Barker R, Jansen RJ, Green SJ, Weging S, Kelliher J, Singh NK, Bezdan D, Galazska J, Brereton NJB. Gonzalez E, et al. NPJ Biofilms Microbiomes. 2024 Aug 29;10(1):71. doi: 10.1038/s41522-024-00545-1. NPJ Biofilms Microbiomes. 2024. PMID: 39209868 Free PMC article. - Obesity I: Overview and molecular and biochemical mechanisms.
Lustig RH, Collier D, Kassotis C, Roepke TA, Kim MJ, Blanc E, Barouki R, Bansal A, Cave MC, Chatterjee S, Choudhury M, Gilbertson M, Lagadic-Gossmann D, Howard S, Lind L, Tomlinson CR, Vondracek J, Heindel JJ. Lustig RH, et al. Biochem Pharmacol. 2022 May;199:115012. doi: 10.1016/j.bcp.2022.115012. Epub 2022 Apr 5. Biochem Pharmacol. 2022. PMID: 35393120 Free PMC article. Review. - SerpinA3N deficiency attenuates steatosis and enhances insulin signaling in male mice.
Tran M, Mostofa G, Picard M, Wu J, Wang L, Shin DJ. Tran M, et al. J Endocrinol. 2023 Feb 3;256(3):e220073. doi: 10.1530/JOE-22-0073. Print 2023 Mar 1. J Endocrinol. 2023. PMID: 36625462 Free PMC article.
References
- IDF Diabetes Atlas. International Diabetes Federation. 7 Brussels, Belgium: 2015.
- Li R, Lu W, Jia J, et al. Relationships between indices of obesity and its cardiovascular comorbidities in a Chinese population. Circ J. 2008;72:973–8. - PubMed
- The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation; Brussels, Belgium: 2005. Apr 14, http://www.idf.org/webdata/docs/Metac_syndrome_def.pdf.
- Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab. 2014;25:255–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK043051/DK/NIDDK NIH HHS/United States
- R01 DK098002/DK/NIDDK NIH HHS/United States
- R01 DK106210/DK/NIDDK NIH HHS/United States
- R37 DK043051/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials