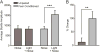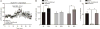Fear conditioning selectively disrupts noradrenergic facilitation of GABAergic inhibition in the basolateral amygdala - PubMed (original) (raw)
Fear conditioning selectively disrupts noradrenergic facilitation of GABAergic inhibition in the basolateral amygdala
M J Skelly et al. Neuropharmacology. 2017 Feb.
Abstract
Inappropriate fear memory formation is symptomatic of many psychopathologies, and delineating the neurobiology of non-pathological fear learning may provide critical insight into treating these disorders. Fear memory formation is associated with decreased inhibitory signaling in the basolateral amygdala (BLA), and disrupted noradrenergic signaling may contribute to this decrease. BLA noradrenergic neurotransmission has been implicated in fear memory formation, and distinct adrenoreceptor (AR) subtypes modulate excitatory and inhibitory neurotransmission in this region. For example, α1-ARs promote GABA release from local inhibitory interneurons, while β3-ARs potentiate neurotransmission at lateral paracapsular (LPC) GABAergic synapses. Conversely, β1/2-ARs amplify excitatory signaling at glutamatergic synapses in the BLA. As increased BLA excitability promotes fear memory formation, we hypothesized that fear learning shifts the balanced regional effects of noradrenergic signaling toward excitation. To test this hypothesis, we used the fear-potentiated startle paradigm in combination with whole cell patch clamp electrophysiology to examine the effects of AR activation on BLA synaptic transmission following fear conditioning in male Long-Evans rats. We first demonstrated that inhibitory neurotransmission is decreased at both local and LPC synapses following fear conditioning. We next measured noradrenergic facilitation of BLA inhibitory signaling at local and LPC synapses using α1-and β3-AR agonists (1 μM A61603 and 10 μM BRL37344), and found that the ability of these agents to facilitate inhibitory neurotransmission is disrupted following fear conditioning. Conversely, we found that fear learning does not disrupt noradrenergic modulation of glutamatergic signaling via a β1/2-AR agonist (1 μM isoproterenol). Taken together, these studies suggest that fear learning increases BLA excitability by selectively disrupting the inhibitory effects of noradrenaline.
Keywords: Fear-potentiated startle; GABA release; Norepinephrine; α1 Adrenoreceptor; β1/2 Adrenoreceptor; β3 Adrenoreceptor.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Figure 1
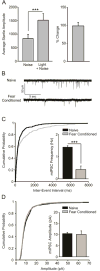
Fear conditioning decreases the frequency of miniature inhibitory post-synaptic currents (mIPSCs) at local GABAergic synapses. A) Fear-conditioned animals (n = 8) exhibited a potentiated startle response to a CS-paired startle-inducing noise burst, indicating acquisition of fear learning (p < 0.001). B) Representative traces of mIPSCs recorded in BLA slices from naïve and fear-conditioned animals. C) Cumulative probability plot demonstrating that mIPSC frequency is lower in slices obtained from fear conditioned animals (bar graph inset, p < 0.001). D) Cumulative probability plot demonstrating that mIPSC amplitude does not differ in slices obtained from naïve and fear conditioned animals (bar graph inset, p > 0.05).
Figure 2
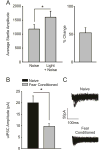
Fear conditioning decreases the amplitude of unitary inhibitory post-synaptic currents (uIPSCs) at LPC synapses. A) Fear-conditioned animals (n = 5) exhibited a potentiated startle response to a CS-paired startle-inducing noise burst, indicating acquisition of fear learning (p < 0.05). B) The average amplitude of LPC uIPSCs is significantly decreased in slices obtained from fear conditioned animals (n = 5 cells/5 rats), as opposed to naïve controls (n = 5 cells/5 rats, p < 0.05). C) Representative traces illustrating that the amplitude of uIPSCs is decreased in fear conditioned animals, as opposed to naïve controls.
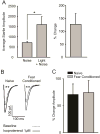
Figure 3
Fear conditioning, but not unpaired shock/light presentation, induces a fear-potentiated startle response. A) Animals exposed to 10 unpaired shock and light presentations do not exhibit a potentiated startle response to a light-paired noise burst (n = 8, p > 0.05), while animals exposed to paired shock/light presentations do exhibit fear-potentiated startle (n = 8, p < 0.001). B) The percent change in average startle amplitude exhibited in response to a light-paired noise is significantly increased in fear conditioned animals as compared to unpaired shock/light controls (p < 0.01).
Figure 4
Fear conditioning decreases α1-AR facilitation of spontaneous inhibitory post-synaptic currents (sIPSCs) at local GABAergic synapses. A) The α1-AR agonist, A61603 (1μM), facilitates presynaptic GABA release from local GABAergic synapses in BLA slices taken from naïve (n = 8 cells/7 animals, p < 0.01) and unpaired (n = 8 cells/8 animals, p < 0.01), but not fear conditioned animals (n = 8 cells/8 animals, p > 0.05). B) Activation of α1-ARs has no effect on sIPSC amplitude across any condition (p > 0.05). C) Representative traces demonstrating the increased frequency of sIPSCs in response to A61603 application in slices taken from naïve animals and unpaired shock/light controls, but not fear conditioned animals. D) Cumulative probability plot demonstrating that sIPSC frequency is significantly decreased in slices obtained from fear conditioned animals, as compared to naïve and unpaired controls (p > 0.001). E) Cumulative probability plot demonstrating that activation of α1-ARs via application of the agonist A61603 results in potentiation of the frequency of sIPSCs in slices obtained from naïve animals and unpaired controls, but not in slices taken from fear conditioned animals. F) The percent increase in sIPSC frequency in response to A61603 application is significantly increased in slices from naïve animals, as compared to animals that underwent fear conditioning (p > 0.05). G) Cumulative probability plots indicating that sIPSC amplitude is equivalent in slices obtained from fear conditioned animals and both naïve and unpaired controls. H) Cumulative probability plot demonstrating that activation of α1-ARs has no effect on sIPSC amplitude in any condition. I) The percent change in the amplitude of sIPSCs in response to activation of α1-ARs via application of A61603 does not differ across conditions (p > 0.05).
Figure 5
Fear conditioning decreases β3-AR facilitation of eIPSCs. A) Timecourse graph demonstrating that application of the β3-AR agonist, BRL37344 (10μM), potentiates evoked inhibitory post-synaptic current (eIPSC) amplitude in slices obtained from naïve animals and UP controls. B) Application of the β3-AR agonist BRL37344 facilitates LPC eIPSC amplitude in slices obtained from naïve (n = 6 cells/animals, p < 0.01) and UP animals (n = 6 cells/animals, p < 0.05), but this facilitation was abolished in slices taken from fear conditioned animals (n = 6 cells/animals, p > 0.05). C) The percent change in eIPSC amplitude in the presence of BRL37344 was greater in naïve and UP slices, as compared to slices taken from FC animals (p < 0.05).
Figure. 6
Fear conditioning does not alter β1/2-AR facilitation of evoked excitatory post-synaptic currents (eEPSCs). A) Fear conditioning resulted in a significant potentiation of the acoustic startle response (n = 7, p < 0.05). B) Representative traces demonstrating that application of the β1/2-AR agonist isoproterenol (1μM) potentiates the amplitude of evoked excitatory post-synaptic currents in fear conditioned animals (n = 9 cells/7 animals, p > 0.01) and naïve controls (n = 12 cells/7 animals, p > 0.01). C) Isoproterenol-induced potentiation of eIPSC amplitude does not differ between naïve and fear conditioned animals (p > 0.05).
Similar articles
- Behavioral and neurophysiological evidence that lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the acquisition and extinction of fear learning.
Skelly MJ, Chappell AM, Ariwodola OJ, Weiner JL. Skelly MJ, et al. Neurobiol Learn Mem. 2016 Jan;127:10-6. doi: 10.1016/j.nlm.2015.11.006. Epub 2015 Nov 28. Neurobiol Learn Mem. 2016. PMID: 26593151 Free PMC article. - Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation.
Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Silberman Y, et al. Neuropsychopharmacology. 2010 Aug;35(9):1886-96. doi: 10.1038/npp.2010.59. Epub 2010 Apr 21. Neuropsychopharmacology. 2010. PMID: 20410872 Free PMC article. - GABAergic Synapses at the Axon Initial Segment of Basolateral Amygdala Projection Neurons Modulate Fear Extinction.
Saha R, Knapp S, Chakraborty D, Horovitz O, Albrecht A, Kriebel M, Kaphzan H, Ehrlich I, Volkmer H, Richter-Levin G. Saha R, et al. Neuropsychopharmacology. 2017 Jan;42(2):473-484. doi: 10.1038/npp.2016.205. Epub 2016 Sep 16. Neuropsychopharmacology. 2017. PMID: 27634356 Free PMC article. - Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders.
Aroniadou-Anderjaska V, Qashu F, Braga MF. Aroniadou-Anderjaska V, et al. Amino Acids. 2007;32(3):305-15. doi: 10.1007/s00726-006-0415-x. Epub 2006 Oct 18. Amino Acids. 2007. PMID: 17048126 Review. - The basolateral amygdala γ-aminobutyric acidergic system in health and disease.
Prager EM, Bergstrom HC, Wynn GH, Braga MF. Prager EM, et al. J Neurosci Res. 2016 Jun;94(6):548-67. doi: 10.1002/jnr.23690. Epub 2015 Nov 19. J Neurosci Res. 2016. PMID: 26586374 Free PMC article. Review.
Cited by
- Impact of Chronic BDNF Depletion on GABAergic Synaptic Transmission in the Lateral Amygdala.
Meis S, Endres T, Munsch T, Lessmann V. Meis S, et al. Int J Mol Sci. 2019 Sep 3;20(17):4310. doi: 10.3390/ijms20174310. Int J Mol Sci. 2019. PMID: 31484392 Free PMC article. - β-Adrenoceptor Blockade in the Basolateral Amygdala, But Not the Medial Prefrontal Cortex, Rescues the Immediate Extinction Deficit.
Giustino TF, Seemann JR, Acca GM, Goode TD, Fitzgerald PJ, Maren S. Giustino TF, et al. Neuropsychopharmacology. 2017 Dec;42(13):2537-2544. doi: 10.1038/npp.2017.89. Epub 2017 May 2. Neuropsychopharmacology. 2017. PMID: 28462941 Free PMC article. - Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior.
McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR. McCall JG, et al. Elife. 2017 Jul 14;6:e18247. doi: 10.7554/eLife.18247. Elife. 2017. PMID: 28708061 Free PMC article. - α1-Adrenergic Receptors in Neurotransmission, Synaptic Plasticity, and Cognition.
Perez DM. Perez DM. Front Pharmacol. 2020 Sep 29;11:581098. doi: 10.3389/fphar.2020.581098. eCollection 2020. Front Pharmacol. 2020. PMID: 33117176 Free PMC article. Review. - Neurobiology of fear and specific phobias.
Garcia R. Garcia R. Learn Mem. 2017 Aug 16;24(9):462-471. doi: 10.1101/lm.044115.116. Print 2017 Sep. Learn Mem. 2017. PMID: 28814472 Free PMC article. Review.
References
- Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH, Li H. beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res. 2008;1209:65–73. - PubMed
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. - PubMed
MeSH terms
Substances
Grants and funding
- R37 AA010422/AA/NIAAA NIH HHS/United States
- T32 GM008464/GM/NIGMS NIH HHS/United States
- P01 AA021099/AA/NIAAA NIH HHS/United States
- R37 AA017531/AA/NIAAA NIH HHS/United States
- R01 AA010422/AA/NIAAA NIH HHS/United States
- F31 AA022275/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials