Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry - PubMed (original) (raw)
Clinical Isolates of Human Coronavirus 229E Bypass the Endosome for Cell Entry
Kazuya Shirato et al. J Virol. 2016.
Abstract
Human coronavirus 229E (HCoV-229E), a causative agent of the common cold, enters host cells via two distinct pathways: one is mediated by cell surface proteases, particularly transmembrane protease serine 2 (TMPRSS2), and the other by endosomal cathepsin L. Thus, specific inhibitors of these proteases block virus infection. However, it is unclear which of these pathways is actually utilized by HCoV-229E in the human respiratory tract. Here, we examined the mechanism of cell entry used by a pseudotyped virus bearing the HCoV-229E spike (S) protein in the presence or absence of protease inhibitors. We found that, compared with a laboratory strain isolated in 1966 and passaged for a half century, clinical isolates of HCoV-229E were less likely to utilize cathepsin L; rather, they showed a preference for TMPRSS2. Two amino acid substitutions (R642M and N714K) in the S protein of HCoV-229E clinical isolates altered their sensitivity to a cathepsin L inhibitor, suggesting that these amino acids were responsible for cathepsin L use. After 20 passages in HeLa cells, the ability of the isolate to use cathepsin increased so that it was equal to that of the laboratory strain; this increase was caused by an amino acid substitution (I577S) in the S protein. The passaged virus showed a reduced ability to replicate in differentiated airway epithelial cells cultured at an air-liquid interface. These results suggest that the endosomal pathway is disadvantageous for HCoV-229E infection of human airway epithelial cells; therefore, clinical isolates are less able to use cathepsin.
Importance: Many enveloped viruses enter cells through endocytosis. Viral spike proteins drive the fusion of viral and endosomal membranes to facilitate insertion of the viral genome into the cytoplasm. Human coronavirus 229E (HCoV-229E) utilizes endosomal cathepsin L to activate the spike protein after receptor binding. Here, we found that clinical isolates of HCoV-229E preferentially utilize the cell surface protease TMPRSS2 rather than endosomal cathepsin L. The endosome is a main site of Toll-like receptor recognition, which then triggers an innate immune response; therefore, HCoV-229E presumably evolved to bypass the endosome by entering the cell via TMPRSS2. Thus, the virus uses a simple mechanism to evade the host innate immune system. Therefore, therapeutic agents for coronavirus-mediated diseases, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), should target cell surface TMPRSS2 rather than endosomal cathepsin.
Keywords: TMPRSS2; cathepsin; coronavirus; endosomes.
Copyright © 2016 American Society for Microbiology.
Figures
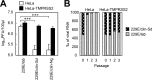
FIG 1
Replication of HCoV-229Es in HeLa and HeLa-TMPRSS2 cells. (A) Viral replication. HeLa or HeLa-TMPRSS2 cells (105) were inoculated with 229E/lab, 229E/clin-Sd, and 229E/clin-Ng strains (103 PFU; n = 12). After 24 h, cells were collected and ultrasonicated, and the virus titer was determined in HeLa cells cultured in medium supplemented with trypsin. The bars and error bars indicate the means and standard deviations (SD), respectively. The data were analyzed using a two-tailed Student t test. (B) Viral replication competition. HeLa or HeLa-TMPRSS2 cells (105) were inoculated with a mixture of 229E/lab and 229E/clin-Sd (103 PFU of each virus) and incubated for 24 h. After passaging three times, viral RNA was quantified in a dual quantitative PCR. The data are expressed as the means of three replicates (n = 3 independent culture wells). *** (very highly significant), P ≤ 0.001.
FIG 2
Blockade of pseudotyped-virus entry by protease inhibitors. HeLa or HeLa-TMPRSS2 cells were inoculated with the VSV-pseudotyped viruses bearing the 229E/lab, 229E/clin-Sd, and 229E/clin-Ng S proteins or the G protein of VSV. (A) Concentration dependency of inhibitors. HeLa or HeLa-TMPRSS2 cells were infected with VSV-pseudotyped viruses in the presence of a serially diluted cathepsin inhibitor, E64d, or a TMPRSS2 inhibitor, camostat. The error bars indicate SD. (B) Blockade of viral entry via two distinct pathways. Cells were infected with VSV-pseudotyped viruses bearing the S protein, as described above, in the presence of 10 μM E64d, 10 μM camostat, or a combination of the two. The GFP-positive cells were counted at 24 h postinfection (n = 6), and the data were expressed as percentages relative to those for the controls (absence of inhibitors). At least 200 GFP-positive cells were counted under control conditions. (C) Cell entry kinetics of pseudotyped HCoV-229Es. After incubation for the indicated times (0, 10, 20, 40, 60, 120, or 240 min), the cells were treated with 10 μM E64d and 10 μM camostat to stop viral entry. At least 200 GFP-positive cells/well were counted at 20 h postinfection (n = 6). The data are expressed as percentages relative to those in HeLa-TMPRSS2 cells cultured in the absence of inhibitors. The asterisks indicate the statistical significance of the data from 229E/clins compared with that from 229E/lab. * (significant), P ≤ 0.05; ** (highly significant), P ≤ 0.01.
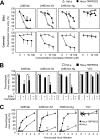
FIG 3
Cathepsin usage by the HCoV-229E spike protein. (A) Effects of amino acid substitutions in the S protein on virus entry. Five amino acid substitutions (R642M, T681R, N714K, V765A, and A775S) are present around the fusion peptide sequence in the S protein of 229E/lab and 229E/clin-Sd. VSV-pseudotyped viruses bearing mutated S proteins or VSV-G were inoculated onto HeLa or HeLa-TMPRSS2 cells, and the GFP-positive cells were counted at 24 h postinfection (n = 6). The data are expressed as percentages relative to those in HeLa-TMPRSS2 cells. (B) Effects of protease inhibitors on pseudotyped viruses bearing S proteins harboring R642M and N714K. HeLa or HeLa-TMPRSS2 cells were inoculated with VSV-pseudotyped viruses in the presence of inhibitors of cathepsin L (CatL) (cathepsin inhibitor III) or cathepsin B (CatB) (CA-074) (each at 10 μM). DMSO-treated cells served as a negative control. At least 200 GFP-positive cells/well were counted at 20 h postinfection (n = 6). The data are expressed as percentages relative to those in HeLa cells treated with DMSO. n.s. (not significant), P > 0.05; * (significant), P ≤ 0.05; ** (highly significant), P ≤ 0.01. The error bars indicate SD.
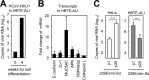
FIG 4
Replication and cell entry by the passaged HCoV-229E clinical isolate. (A) Replication of the passage 20 clinical isolate. HeLa or HeLa-TMPRSS2 cells (105) were inoculated with 229E/lab, 229E/clin-Sd-p1, and 229E/clin-Sd-p20 (103 PFU) (n = 6). After 24 h, the cells were collected and ultrasonicated, and virus titers were determined in HeLa cells cultured in medium supplemented with trypsin. P1, passage 1; P20, passage 20. (B) Effects of amino acid substitutions in the S protein on virus entry. There was one amino acid mutation in the S protein of 229E/clin-Sd at passage 20 (I577S). HeLa or HeLa-TMPRSS2 cells were inoculated with VSV-pseudotyped viruses bearing HCoV-229E S proteins or the VSV G protein in the presence or absence of 10 μM cathepsin inhibitor III, and at least 200 GFP-positive cells/well were counted under control conditions at 24 h postinfection (n = 4). The data are expressed as percentages relative to those in HeLa-TMPRSS2 cells. ** (highly significant), P ≤ 0.01; *** (very highly significant), P ≤ 0.001. The error bars indicate SD.
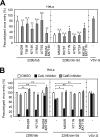
FIG 5
Replication of the passaged HCoV-229E clinical isolate in HBTE-ALI cells. (A) Characterization of HBTE cells by HCoV-HKU-1 infection. HBTE cells were cultured for 4 weeks in differentiation medium at an air-liquid interface (HBTE-ALI) in a 6.5-mm-diameter Transwell chamber. To confirm differentiation, HBTE cells, differentiated (incubated for 4 weeks) or undifferentiated (incubated for 0 weeks), were inoculated with HCoV-HKU1 (n = 2). After 2 h, the inoculated virus was removed and the cells were washed three times with medium. After 72 h of incubation at the ALI, RNA was collected from the medium, and real-time PCR was performed to quantify viral RNA. (B) Characterization of HBTE cells by measurement of cellular transcripts. Expression of cellular mRNAs encoding cell differentiation markers (E-cadherin, ZO-1, and MUC5AC) and factors associated with HCoV-229E infection (APN, TMPRSS2, and cathepsin L) in differentiated and undifferentiated HBTE cells was measured by real-time PCR (n = 4). The data are expressed as the fold change in transcript levels in differentiated cells relative to that in undifferentiated cells. (C) Replication of passaged HCoV-229E in HBTE cells. HBTE-ALI cells were inoculated with 229E/clin-Sd-p1 and 229E/clin-Sd-p20 (104 PFU), the titers of which were measured in HeLa-TMPRSS2 cells cultured in medium supplemented with trypsin (n = 4). HeLa cells were used as a control to confirm differential RNA expression due to the differing abilities of passage 1 and passage 20 virus to use cathepsin. After 1 h, the inoculated virus was removed, and the cells were washed three times in culture medium. After 24 h of incubation at the ALI, cellular RNA was collected, and viral RNA was measured by real-time PCR. ** (highly significant), P ≤ 0.01; *** (very highly significant), P ≤ 0.001. The error bars indicate SD.
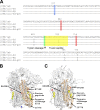
FIG 6
Homology modeling of HCoV-229E S protein. (A) Alignment of HCoV-229E S proteins around a fusion peptide. The intermediate regions between the S1 and S2 subunits of the HCoV-229E S glycoproteins were aligned using MAFFT software (CBRC, Japan). The trypsin cleavage site and the fusion peptide are indicated in green and yellow, respectively. The positions of the mutations causing an increase or decrease in cathepsin use are indicated in red or blue, respectively. (B and C) Theoretical structures of the S protein. The structures of HCoV-229E were constructed using MOE software based on the cryoelectron microscopic structure of the MHV S protein (B) or the HCoV-HKU1 S protein (C) in the prefusion state. The region around the fusion peptide in the S2 subunit (residues 572 to 953) is shown in orange. Mutations that cause increased or decreased cathepsin usage are shown in blue or red, respectively. The putative fusion peptide and the trypsin cleavage site are shown in yellow and green.
Similar articles
- TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium.
Bertram S, Dijkman R, Habjan M, Heurich A, Gierer S, Glowacka I, Welsch K, Winkler M, Schneider H, Hofmann-Winkler H, Thiel V, Pöhlmann S. Bertram S, et al. J Virol. 2013 Jun;87(11):6150-60. doi: 10.1128/JVI.03372-12. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536651 Free PMC article. - Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry.
Shirato K, Kawase M, Matsuyama S. Shirato K, et al. Virology. 2018 Apr;517:9-15. doi: 10.1016/j.virol.2017.11.012. Epub 2017 Dec 6. Virology. 2018. PMID: 29217279 Free PMC article. - Functional analysis of potential cleavage sites in the MERS-coronavirus spike protein.
Kleine-Weber H, Elzayat MT, Hoffmann M, Pöhlmann S. Kleine-Weber H, et al. Sci Rep. 2018 Nov 9;8(1):16597. doi: 10.1038/s41598-018-34859-w. Sci Rep. 2018. PMID: 30413791 Free PMC article. - Cathepsin L, transmembrane peptidase/serine subfamily member 2/4, and other host proteases in COVID-19 pathogenesis - with impact on gastrointestinal tract.
Berdowska I, Matusiewicz M. Berdowska I, et al. World J Gastroenterol. 2021 Oct 21;27(39):6590-6600. doi: 10.3748/wjg.v27.i39.6590. World J Gastroenterol. 2021. PMID: 34754154 Free PMC article. Review. - Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis.
Millet JK, Whittaker GR. Millet JK, et al. Virus Res. 2015 Apr 16;202:120-34. doi: 10.1016/j.virusres.2014.11.021. Epub 2014 Nov 22. Virus Res. 2015. PMID: 25445340 Free PMC article. Review.
Cited by
- Computational and in vitro experimental analyses of the anti-COVID-19 potential of Mortaparib and MortaparibPlus.
Kumar V, Sari AN, Meidinna HN, Dhanjal JK, Subramani C, Basu B, Kaul SC, Vrati S, Sundar D, Wadhwa R. Kumar V, et al. Biosci Rep. 2021 Oct 29;41(10):BSR20212156. doi: 10.1042/BSR20212156. Biosci Rep. 2021. PMID: 34647577 Free PMC article. - Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells.
Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, Shimotai Y, Momma H, Ichinose M, Kawase T. Yamaya M, et al. Respir Investig. 2020 May;58(3):155-168. doi: 10.1016/j.resinv.2019.12.005. Epub 2020 Feb 21. Respir Investig. 2020. PMID: 32094077 Free PMC article. - Design of Potent Membrane Fusion Inhibitors against SARS-CoV-2, an Emerging Coronavirus with High Fusogenic Activity.
Zhu Y, Yu D, Yan H, Chong H, He Y. Zhu Y, et al. J Virol. 2020 Jul 1;94(14):e00635-20. doi: 10.1128/JVI.00635-20. Print 2020 Jul 1. J Virol. 2020. PMID: 32376627 Free PMC article. - TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells.
Bestle D, Heindl MR, Limburg H, Van Lam van T, Pilgram O, Moulton H, Stein DA, Hardes K, Eickmann M, Dolnik O, Rohde C, Klenk HD, Garten W, Steinmetzer T, Böttcher-Friebertshäuser E. Bestle D, et al. Life Sci Alliance. 2020 Jul 23;3(9):e202000786. doi: 10.26508/lsa.202000786. Print 2020 Sep. Life Sci Alliance. 2020. PMID: 32703818 Free PMC article. - Single Amino Acid Substitution in the Receptor Binding Domain of Spike Protein Is Sufficient To Convert the Neutralization Profile between Ethiopian and Middle Eastern Isolates of Middle East Respiratory Coronavirus.
Sugimoto S, Kakizaki M, Kawase M, Kawachi K, Ujike M, Kamitani W, Sentsui H, Shirato K. Sugimoto S, et al. Microbiol Spectr. 2023 Feb 6;11(2):e0459022. doi: 10.1128/spectrum.04590-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36744940 Free PMC article.
References
- Shirato K, Kawase M, Watanabe O, Hirokawa C, Matsuyama S, Nishimura H, Taguchi F. 2012. Differences in neutralizing antigenicity between laboratory and clinical isolates of HCoV-229E isolated in Japan in 2004-2008 depend on the S1 region sequence of the spike protein. J Gen Virol 93:1908–1917. doi:10.1099/vir.0.043117-0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous