PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma - PubMed (original) (raw)
PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma
Xi-Wen Bi et al. J Hematol Oncol. 2016.
Abstract
Background: Natural killer/T-cell lymphoma (NKTCL) is an Epstein-Barr virus (EBV)-associated, highly aggressive lymphoma. Treatment outcome remains sub-optimal, especially for advanced-stage or relapsed diseases. Programmed cell death receptor 1 (PD-1) and PD ligand 1 (PD-L1) have become promising therapeutic targets for various malignancies, but their role in the pathogenesis and their interactions with EBV in NKTCL remains to be investigated.
Methods: Expression of PD-L1 was measured in NK-92 (EBV-negative) and SNK-6 (EBV-positive) cells by western blot, quantitative real-time PCR and enzyme-linked immunosorbent assay, and flow cytometry, respectively. Latent membrane protein 1 (LMP1)-harboring lentiviral vectors were transfected into NK-92 cells to examine the correlation between LMP1 and PD-L1 expression. Proteins in the downstream pathways of LMP1 signaling were measured in NK-92 cells transfected with LMP1-harboring or negative control vectors as well as in SNK-6 cells. PD-L1 expression on tumor specimens and serum concentration of soluble PD-L1 were collected in a retrospective cohort of patients with Ann Arbor stage I~II NKTCL, and their prognostic significance were analyzed.
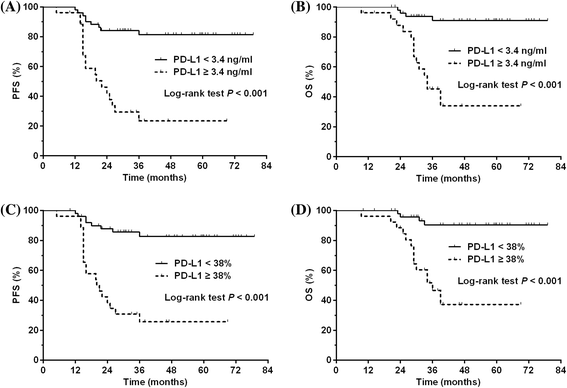
Results: Expression of PD-L1 was significantly higher in SNK-6 cells than in NK-92 cells, at both protein and mRNA levels. Expression of PD-L1 was remarkably upregulated in NK-92 cells transfected with LMP1-harboring lentiviral vectors compared with those transfected with negative control vectors. Proteins in the MAPK/NF-κB pathway were upregulated in LMP1-expressing NK-92 cells compared with the negative control. Selective inhibitors of those proteins induced significant downregulation of PD-L1 expression in LMP1-expressing NK-92 cells as well as in SNK-6 cells. Patients with a high concentration of serum soluble PD-L1 (≥3.4 ng/ml) or with a high percentage of PD-L1 expression in tumor specimens (≥38 %) exhibited significantly lower response rate to treatment and remarkably worse survival, compared with their counterparts. A high concentration of serum soluble PD-L1 and a high percentage of PD-L1 expression in tumor specimens were independent adverse prognostic factors among patients with stage I~II NKTCL.
Conclusions: PD-L1 expression positively correlated LMP1 expression in NKTCL, which was probably mediated by the MAPK/NF-κB pathway. PD-L1 expression in serum and tumor tissues has significant prognostic value for early-stage NKTCL.
Keywords: Epstein–Barr virus; Latent membrane protein 1; Natural killer/T-cell lymphoma; Programmed cell death receptor 1.
Figures
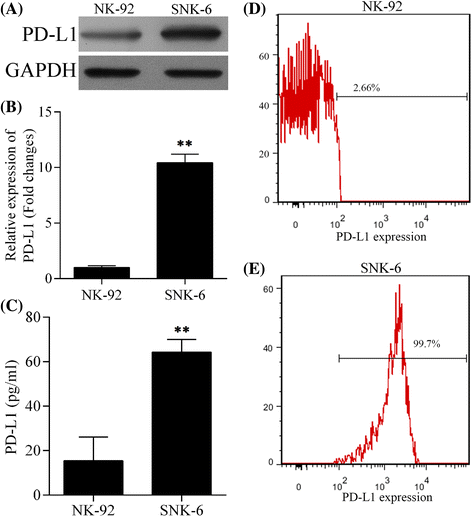
Fig. 1
Expression of PD-L1 in NK cell line NK-92 (EBV-negative) and NKTCL cell line SNK-6 (EBV-positive). The level of a PD-L1 protein detected by western blot, b PD-L1 mRNA detected by quantitative real-time PCR, c soluble PD-L1 protein in cell culture supernatant detected by ELISA, and d, e PD-L1 expression on cell surface detected by flow cytometry in NK-92 and SNK-6 cell lines, respectively. ** P < 0.05
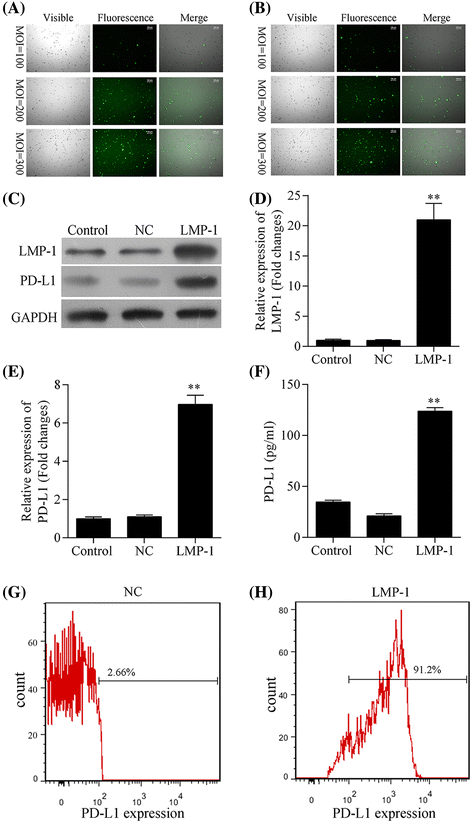
Fig. 2
Expression of PD-L1 was upregulated by LMP1 in NK-92 cells. a, b Infection efficiency of LV5-LMP1 vector (a) and LV5 vector (LV5-NC, b) in NK-92 cell line at a multiplicity of infection (MOI) of 100, 200, and 300, respectively. The level of c LMP1 and PD-L1 proteins detected by western blot, d LMP1 and e PD-L1 mRNA detected by quantitative real-time PCR, f soluble PD-L1 protein in cell culture supernatant detected by ELISA, and g, h PD-L1 expression on cell surface detected by flow cytometry in LMP1-expressing NK92 (LMP1) and negative control NK92 (NC) cell lines, respectively. ** P < 0.05
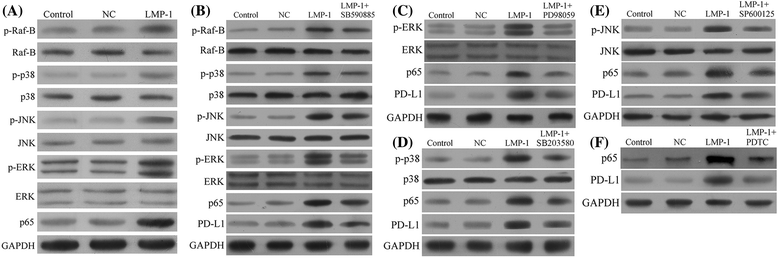
Fig. 3
LMP1 upregulated PD-L1 expression through MAPK/NF-κB pathway in NK92 cells. a The expression level of p-Raf-B, Raf-B, p-p38, p38, p-JNK, JNK, p-ERK, ERK, and p65 in LMP1-expressing NK92 (LMP1) and negative control NK92 (NC) cell lines. b The expression level of p-Raf-B, Raf-B, p-p38, p38, p-JNK, JNK, p-ERK, ERK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 0.1 μM SB590885, a selective B-Raf inhibitor for 1 h. c The expression level of p-ERK, ERK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 20 μM PD98059, a selective ERK inhibitor for 1 h. d The expression level of p-p38, p38, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 10 μM SB203580, a selective p38 inhibitor for 1 h. e The expression level of p-JNK, JNK, p65, and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 20 μM SP600125, a selective JNK inhibitor for 1 h. f The expression level of p65 and PD-L1 in negative control NK92 (NC) or LMP1-expressing NK92 (LMP1) cell line treated with 100 μM pyrrolidine dithiocarbamate (PDTC), a selective inhibitor of NF-κB for 1 h
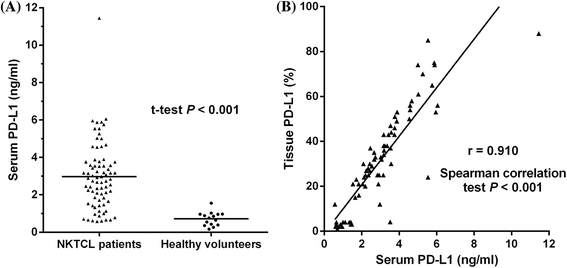
Fig. 4
a Concentration of serum soluble PD-L1 in patients with natural killer/T-cell lymphoma (NKTCL) and healthy individuals. b Correlation between concentration of serum soluble PD-L1 (ng/ml) and PD-L1 expression in tumor tissues (%) in patients with NKTCL
Fig. 5
a Progression-free survival (PFS) for NKTCL patients with a serum soluble PD-L1 of <3.4 and ≥3.4 ng/ml. b Overall survival (OS) for NKTCL patients with a serum soluble PD-L1 of <3.4 and ≥3.4 ng/ml. c PFS for NKTCL patients with a histological PD-L1 expression of <38 and ≥38 %. c OS for NKTCL patients with a histological PD-L1 expression of <38 and ≥38 %
Similar articles
- IL-2Rα up-regulation is mediated by latent membrane protein 1 and promotes lymphomagenesis and chemotherapy resistance in natural killer/T-cell lymphoma.
Wang L, Bi XW, Zhu YJ, He YZ, Lai QY, Xia ZJ, Cai QQ. Wang L, et al. Cancer Commun (Lond). 2018 Oct 19;38(1):62. doi: 10.1186/s40880-018-0334-8. Cancer Commun (Lond). 2018. PMID: 30340635 Free PMC article. - Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis.
Kim WY, Jung HY, Nam SJ, Kim TM, Heo DS, Kim CW, Jeon YK. Kim WY, et al. Virchows Arch. 2016 Nov;469(5):581-590. doi: 10.1007/s00428-016-2011-0. Epub 2016 Sep 5. Virchows Arch. 2016. PMID: 27595782 - LMP1 enhances aerobic glycolysis in natural killer/T cell lymphoma.
Song W, Gao Y, Wu J, Li H, Shi Z, Gong C, Zhang Z, Li Z, Zhang M. Song W, et al. Cell Death Dis. 2024 Aug 20;15(8):604. doi: 10.1038/s41419-024-06999-7. Cell Death Dis. 2024. PMID: 39164228 Free PMC article. - Updating targets for natural killer/T-cell lymphoma immunotherapy.
Xue W, Zhang M. Xue W, et al. Cancer Biol Med. 2021 Feb 15;18(1):52-62. doi: 10.20892/j.issn.2095-3941.2020.0400. Cancer Biol Med. 2021. PMID: 33628584 Free PMC article. Review. - Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma.
Xie M, Huang X, Ye X, Qian W. Xie M, et al. Int Immunopharmacol. 2019 Dec;77:105999. doi: 10.1016/j.intimp.2019.105999. Epub 2019 Nov 6. Int Immunopharmacol. 2019. PMID: 31704289 Review.
Cited by
- Overexpression of S100A9 in tumor stroma contribute to immune evasion of NK/T cell lymphoma and predict poor response rate.
Zhou Z, Chen X, Li Z, Wang X, Zhang M. Zhou Z, et al. Sci Rep. 2021 May 27;11(1):11220. doi: 10.1038/s41598-021-90794-3. Sci Rep. 2021. PMID: 34045609 Free PMC article. - Epstein-Barr Virus LMP1 Induces Soluble PD-L1 in Nasopharyngeal Carcinoma.
Kase K, Kondo S, Wakisaka N, Dochi H, Mizokami H, Kobayashi E, Kano M, Komori T, Hirai N, Ueno T, Nakanishi Y, Hatano M, Endo K, Moriyama-Kita M, Sugimoto H, Yoshizaki T. Kase K, et al. Microorganisms. 2021 Mar 15;9(3):603. doi: 10.3390/microorganisms9030603. Microorganisms. 2021. PMID: 33804064 Free PMC article. - Non-apoptotic activity of the mitochondrial protein SMAC/Diablo in lung cancer: Novel target to disrupt survival, inflammation, and immunosuppression.
Pandey SK, Shteinfer-Kuzmine A, Chalifa-Caspi V, Shoshan-Barmatz V. Pandey SK, et al. Front Oncol. 2022 Sep 14;12:992260. doi: 10.3389/fonc.2022.992260. eCollection 2022. Front Oncol. 2022. PMID: 36185255 Free PMC article. - Programmed Cell Death-Ligand 1 in Head and Neck Squamous Cell Carcinoma: Molecular Insights, Preclinical and Clinical Data, and Therapies.
Meliante PG, Barbato C, Zoccali F, Ralli M, Greco A, de Vincentiis M, Colizza A, Petrella C, Ferraguti G, Minni A, Fiore M. Meliante PG, et al. Int J Mol Sci. 2022 Dec 6;23(23):15384. doi: 10.3390/ijms232315384. Int J Mol Sci. 2022. PMID: 36499710 Free PMC article. Review. - Metformin attenuates PD-L1 expression through activating Hippo signaling pathway in colorectal cancer cells.
Zhang JJ, Zhang QS, Li ZQ, Zhou JW, Du J. Zhang JJ, et al. Am J Transl Res. 2019 Nov 15;11(11):6965-6976. eCollection 2019. Am J Transl Res. 2019. PMID: 31814900 Free PMC article.
References
- Chan JK, Quintanilla-Martinez L, Ferry JA, Peh S-C, et al. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 285–288.
- Chan JK, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2001. pp. 204–207.
- Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. - DOI - PubMed
- Bi XW, Li YX, Fang H, Jin J, Wang WH, Wang SL, et al. High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer’s ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys. 2013;87:1086–1093. doi: 10.1016/j.ijrobp.2013.08.040. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials