Autophagy is involved in aldosterone‑induced mesangial cell proliferation - PubMed (original) (raw)
Autophagy is involved in aldosterone‑induced mesangial cell proliferation
Min Yang et al. Mol Med Rep. 2016 Nov.
Abstract
The aim of the present study was to investigate whether autophagy is involved in aldosterone (Aldo)-induced mesangial cell (MC) proliferation. MCs were incubated with 10‑7 M Aldo for 24 h. Proliferation of MCs, and the underlying mechanisms, were subsequently analyzed using [3H]thymidine assay, cell counting assay, western blotting and RNA interference (RNAi). Aldo was revealed to induce autophagy, as indicated by the increased conversion from microtubule‑associated protein 1A/1B‑light chain 3 (LC3)‑I to LC3‑II, the increased expression levels of autophagy‑related gene 7 (Atg7) and the increased degradation of p62, which was accompanied by MC proliferation. Notably, pharmacological inhibition of autophagy or RNAi‑mediated knockdown of Atg7 attenuated Aldo‑induced MC proliferation, suggesting that autophagy was at least partially responsible for this effect. The results of the present study provided evidence that autophagy is critical for regulating Aldo‑induced MC proliferation.
Figures
Figure 1.
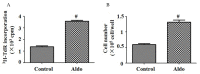
Aldo induced MC proliferation. MC proliferation was evaluated by (A) [3H]thymidine incorporation and (B) cell counting. MCs were treated with 10−7 M Aldo for 24 h. Data are presented as the mean ± standard deviation of six series of experiments. #P<0.05 vs. control. Aldo, aldosterone; MC, mesangial cell.
Figure 2.
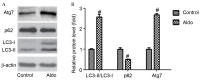
Aldo induced autophagy in MCs. (A) Western blot analysis revealed the protein expression levels of LC3-II/LC3-I, p62, Atg7 and β-actin following treatment with 10−7 M Aldo for 24 h. (B) Quantification of western blotting revealed the increase in LC3-II/LC3-I and Atg7 protein expression levels, and decrease in p62, following Aldo treatment. Results were normalized to β-actin. Data are presented as the mean ± standard deviation of three series of experiments. #P<0.05 vs. control. Aldo, aldosterone; MC, mesangial cell; LC3, microtubule-associated protein 1A/1B-light chain 3; Atg7, autophagy-related gene 7.
Figure 3.
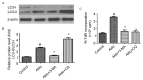
Pharmacologically inhibiting autophagy prevented Aldo-induced MC proliferation. (A) Western blot analysis revealed the protein expression levels of LC3-II/LC3-I and β-actin following treatment with 10−7 M Aldo in the presence or absence of 3-MA or CQ for 24 h. (B) Quantification of western blotting revealed the decrease of LC3-II/LC3-I protein expression levels following 3-MA treatment, and their increase following CQ treatment. Results were normalized to β-actin. Data are presented as the mean ± standard deviation of three series of experiments. (C) MC proliferation was evaluated by [3H]thymidine incorporation following treatment with 10−7 M Aldo in the presence or absence of 3-MA or CQ for 24 h. Treatment with 3-MA or CQ abrogated Aldo-induced proliferation. Data are presented as the mean ± standard deviation of six series of experiments. #P<0.05 vs. control; *P<0.05 vs. Aldo alone. Aldo, aldosterone; MC, mesangial cell; LC3, microtubule-associated protein 1A/1B-light chain 3; Atg7, autophagy-related gene 7; 3-MA, 3-methyladenine; CQ, chloroquine.
Figure 4.
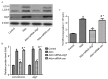
Inhibiting autophagy with siRNA-Atg7 prevented Aldo-induced MC proliferation. (A) MCs were transfected with 20 nM siRNA-Atg7 or siRNA-con as a control. A total of 24 h post-transfection, MCs were treated with 10−7 M Aldo for a further 24 h. Western blot analysis revealed the protein expression levels of LC3-II/LC3-I, Atg7 and β-actin. (B) Quantification of western blotting revealed the decrease in LC3-II/LC3-I and Atg7 protein expression levels following siRNA-Atg7 transfection. Results were normalized to β-actin. Data are presented as the mean ± standard deviation of three series of experiments. (C) MC proliferation was evaluated by [3H]thymidine incorporation following siRNA-Atg7 transfection. Transfection with siRNA-Atg7 abrogated Aldo-induced proliferation of MCs. Data are presented as the mean ± standard deviation of six series of experiments. #P<0.05 vs. control; *P<0.05 vs. Aldo alone. siRNA, small interfering RNA; Atg7, autophagy-related gene 7; Aldo, aldosterone; MC, mesangial cell; con, control; LC3, microtubule-associated protein 1A/1B-light chain 3; con, control.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources