Store-operated interactions between plasmalemmal STIM1 and TRPC1 proteins stimulate PLCβ1 to induce TRPC1 channel activation in vascular smooth muscle cells - PubMed (original) (raw)
. 2017 Feb 15;595(4):1039-1058.
doi: 10.1113/JP273302. Epub 2016 Dec 7.
Affiliations
- PMID: 27753095
- PMCID: PMC5309361
- DOI: 10.1113/JP273302
Store-operated interactions between plasmalemmal STIM1 and TRPC1 proteins stimulate PLCβ1 to induce TRPC1 channel activation in vascular smooth muscle cells
Jian Shi et al. J Physiol. 2017.
Abstract
Key points: Depletion of Ca2+ stores activates store-operated channels (SOCs), which mediate Ca2+ entry pathways that regulate cellular processes such as contraction, proliferation and gene expression. In vascular smooth muscle cells (VSMCs), stimulation of SOCs composed of canonical transient receptor potential channel 1 (TRPC1) proteins requires G protein α q subunit (Gαq)/phospholipase C (PLC)β1/protein kinase C (PKC) activity. We studied the role of stromal interaction molecule 1 (STIM1) in coupling store depletion to this activation pathway using patch clamp recording, GFP-PLCδ1-PH imaging and co-localization techniques. Store-operated TRPC1 channel and PLCβ1 activities were inhibited by STIM1 short hairpin RNA (shRNA) and absent in TRPC1-/- cells, and store-operated PKC phosphorylation of TRPC1 was inhibited by STIM1 shRNA. Store depletion induced interactions between STIM1 and TRPC1, Gαq and PLCβ1, which required STIM1 and TRPC1. Similar effects were produced with noradrenaline. These findings identify a new activation mechanism of TRPC1-based SOCs in VSMCs, and a novel role for STIM1, where store-operated STIM1-TRPC1 interactions stimulate Gαq/PLCβ1/PKC activity to induce channel gating.
Abstract: In vascular smooth muscle cells (VSMCs), stimulation of canonical transient receptor potential channel 1 (TRPC1) protein-based store-operated channels (SOCs) mediates Ca2+ entry pathways that regulate contractility, proliferation and migration. It is therefore important to understand how these channels are activated. Studies have shown that stimulation of TRPC1-based SOCs requires G protein α q subunit (Gαq)/phospholipase C (PLC)β1 activities and protein kinase C (PKC) phosphorylation, although it is unclear how store depletion stimulates this gating pathway. The present study examines this issue by focusing on the role of stromal interaction molecule 1 (STIM1), an endo/sarcoplasmic reticulum Ca2+ sensor. Store-operated TRPC1 channel activity was inhibited by TRPC1 and STIM1 antibodies and STIM1 short hairpin RNA (shRNA) in wild-type VSMCs, and was absent in TRPC1-/- VSMCs. Store-operated PKC phosphorylation of TRPC1 was reduced by knockdown of STIM1. Moreover, store-operated PLCβ1 activity measured with the fluorescent phosphatidylinositol 4,5-bisphosphate/inositol 1,4,5-trisphosphate biosensor GFP-PLCδ1-PH was reduced by STIM1 shRNA and absent in TRPC1-/- cells. Immunocytochemistry, co-immunoprecipitation and proximity ligation assays revealed that store depletion activated STIM1 translocation from within the cell to the plasma membrane (PM) where it formed STIM1-TRPC1 complexes, which then associated with Gαq and PLCβ1. Noradrenaline also evoked TRPC1 channel activity and associations between TRPC1, STIM1, Gαq and PLCβ1, which were inhibited by STIM1 knockdown. Effects of N-terminal and C-terminal STIM1 antibodies on TRPC1-based SOCs and STIM1 staining suggest that channel activation may involve insertion of STIM1 into the PM. The findings of the present study identify a new activation mechanism of TRPC1-based SOCs in VSMCs, and a novel role for STIM1, in which store-operated STIM1-TRPC1 interactions stimulate PLCβ1 activity to induce PKC phosphorylation of TRPC1 and channel gating.
Keywords: PLC; STIM1; TRPC; vascular smooth muscle.
© 2016 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures
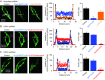
Figure 1. TRPC1 compose SOCs in contractile VSMCs
A, development of a store‐operated whole‐cell current in a mesenteric artery VSMC from a WT mouse following break‐in into the whole‐cell configuration (w.c.) was inhibited by bath application of T1E3. Vertical deflections represent currents evoked by voltage ramps from +100 mV to −150 mV (750 ms duration) every 30 s from a holding potential of 0 mV. Development of a store‐operated whole‐cell TRPC1 current was absent in a TRPC1−/− VMSC. Mean I/V relationships of store‐operated whole‐cell currents demonstrate that T1E3 reduced store‐operated whole‐cell currents at all membrane potentials tested in WT VSMCs and that store‐operated currents were absent in TRPC1−/− VSMCs (each point is at least n = 6). B, BAPTA‐AM evoked single channel activity in a cell‐attached patch (c/a) held at −80 mV from a WT VSMC was maintained following patch excision into the inside‐out configuration (i/o). Bath application of an intracellular acting anti‐TRPC1 antibody to the cytosolic surface of the inside‐out patch inhibited BAPTA‐evoked channel activity. BAPTA‐AM failed to activate channel activity in a cell‐attached held at −80 mV from a TRPC1−/− VSMC. Mean data of the inhibitory effect of the anti‐TRPC1 antibody on BAPTA‐evoked channel activity. Note that channel activities were maintained on changing from cell‐attached to inside‐out patch configurations (n = 7; *** P < 0.001).
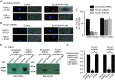
Figure 2. TRPC1‐based SOCs are dependent on STIM1
A, western blots and mean data confirm that two different STIM1 shRNA sequences (shRNA1 and shRNA2) reduced STIM1 expression (three primary rabbit portal vein VSMC culture preparations; ** P < 0.01). B, traces and mean I/V relationships showing that peak amplitude of store‐operated whole‐cell TRPC1‐based currents were greatly reduced at all membrane potentials tested following transduction of rabbit portal vein VSMCs with shRNA sequences compared to scrambled shRNA sequences. In the presence of scrambled sequences, store‐operated TRPC1‐based currents were inhibited by an anti‐STIM1 antibody (n = 6). C, recordings and mean data showing that BAPTA‐AM‐evoked TRPC1‐based SOCs were reduced by shRNA sequences targeting STIM1 compared to scrambled shRNA in VSMCs (n = 6; *** P < 0.001).
Figure 3. Store‐operated phosphorylation of TRPC1 proteins requires STIM1
A, co‐immunoprecipitation of rabbit portal vein tissue lysates with anti‐phosphorylated serine (pSer) and threonine (pThr) antibodies followed by western blotting (WB) with an anti‐TRPC1 antibody shows that basal TRPC1 phosphorylation is increased by pre‐treatment with BAPTA‐AM for 10 min, and that this increase was reduced by STIM1 shRNA1 and coapplication of GF109203X. B, mean relative band densities normalized to BAPTA‐AM bands (three different tissue lysate preparations; * P < 0.05, ** P < 0.01). [Colour figure can be viewed at
wileyonlinelibrary.com
]
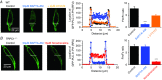
Figure 4. Store‐operated PLC activity is mediated by STIM1
A, image from a single rabbit portal vein VSMC showing that in control conditions the location of GFP‐PLCδ1‐PH‐mediated signals (measured as relative fluorescence units; RFU) was predominantly expressed at the PM (black). In the same cell, pre‐treatment with BAPTA‐AM for 10 min induced translocation of signals to the cytosol (blue), and coapplication of U73122 for 5 min reversed these cytosolic signals back to the PM (orange). Graphs of RFU of line scans for the region denoted along the white dotted lines show GFP‐PLCδ1‐PH signals across the cell width. Mean F m/F c ratios of GFP‐PLCδ1‐PH‐mediated signals represent 20 cells from three different experiments (*** P<0.001). B and C, images and mean data showing that transduction of rabbit portal vein VSMCs with either STIM1 shRNA1 or shRNA2 sequences prevented BAPTA‐AM inducing translocation of GFP‐PLCδ1‐PH signals to the cytosol. Under both these conditions, application of noradrenaline for 5 min (red, applied in the presence of 1 μ
m
Wortmannin to prevent cell contraction) was still able to induce translocation of GFP‐PLCδ1‐PH signals from the PM to the cytosol (20 cells for each STIM1 shRNA sequence from three different experiments; *** P < 0.01).
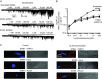
Figure 5. Store‐operated PLC activity requires TRPC1
A, WT mesenteric artery VSMCs: application of BAPTA‐AM for 10 min induced translocation of GFP‐PLCδ1‐PH‐mediated signals from the PM to the cytosol which was prevented by coapplication of U73122 for 5 min (20 cells from three different experiments; *** P < 0.001). B, TRPC1−/− VSMCs: BAPTA‐AM did not alter the cellular distribution of GFP‐PLCδ1‐PH signals whereas application of noradrenaline for 5 min induced translocation of GFP‐PLCδ1‐PH signals from the PM to the cytosol (20 cells from three different experiments; *** P < 0.001).
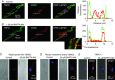
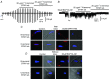
Figure 6. Store‐depletion induces co‐localizations between STIM1 and TRPC1 at the PM
A and B, images from two different rabbit portal vein VSMCs showing TRPC1 (green) and STIM1 (red) staining. Changes in the relative fluorescence of TRPC1 and STIM1 staining across the cell width were determined from the line scan (dotted white line). In a resting cell (A), TRPC1 staining was predominantly present at the PM, whereas labelling for STIM1 was located within the cytosol. In a cell treated with BAPTA‐AM for 10 min (B), both TRPC1 and STIM1 were located at the PM in discrete puncta. Inset: co‐localization between TRPC1 and STIM1 staining (yellow) at the PM. C and D, PLA images showing BAPTA‐AM induced fluorescence signals between STIM1 and TRPC1 which were predominantly at the PM in rabbit portal vein and mice mesenteric artery VSMCs. E, immunocytochemical image showing that, in TRPC1−/− mesenteric artery VSMCs, BAPTA‐AM induced translocation of STIM1 from the cytosol to the PM where it produced uniform, non‐puncta‐like staining.
Figure 7. Store‐depletion evokes associations between TRPC1, STIM1, Gαq and PLCβ1
A, western blots showing that pre‐treatment with BAPTA‐AM for 10 min induced associations between TRPC1 and STIM1, Gαq and PLCβ1, which were reduced by STIM1 shRNA1. Primary cultured rabbit portal vein VSMC lysates were initially immunoprecipitated (IP) with anti‐TRPC1 antibodies and were then western blotted (WB) with anti‐STIM1, anti‐Gαq or anti‐PLCβ antibodies. B, mean data for relative band intensities of BAPTA‐AM‐evoked interactions with TRPC1 shown in A (three different cell lysates; * P < 0.05). C, application of BAPTA‐AM for 10 min did not alter interactions between TRPC6 and STIM1 (left) or change expression levels of TRPC6 (right) in rabbit portal vein tissue lysates. D, primary cultured WT mesenteric artery VSMCs: BAPTA‐AM evoked interactions between STIM1 and TRPC1, Gαq and PLCβ1, which were absent in cell lysates from TRPC1−/− VSMCs.
Figure 8. Store‐operated induced interactions between STIM1, Gαq and PLCβ1 require TRPC1
A, PLA images from WT mesenteric artery VSMCs showing that BAPTA‐AM induced interactions between STIM and Gαq, and also between STIM1 and PLCβ1. B, BAPTA‐AM‐evoked interactions between STIM and Gαq, and STIM1 and PLCβ1 were absent in TRPC1−/− VSMCs.
Figure 9. Store‐operated interactions between TRPC1 and STIM1 do not require PLCβ1
A_–_C, application of BAPTA‐AM for 10 min induced interactions between TRPC1 and PLCβ1 in rabbit portal vein VSMCs measured using PLA that were reduced by expression of PLCβ1 shRNA1 and shRNA2 sequences, whereas associations between TRPC1 and STIM1 were unaffected (three different preparations; * P < 0.05). D and E, BAPTA‐AM‐induced interactions between TRPC1 and PLCβ1 measured using co‐immunoprecipitation were reduced by expression of PLCβ1 shRNA1 and shRNA2 sequences, whereas associations between TRPC1 and STIM1 were unaffected (three different rabbit portal vein cell lysates; * P < 0.05).
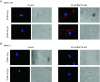
Figure 10. Differential actions of antibodies raised against N‐terminal and C‐terminal regions of STIM1 on activation of TRPC1‐based SOCs
A, original trace showing that a store‐operated whole‐cell current from a WT mesenteric artery VSMC was inhibited by bath application of a N‐terminal but not a C‐terminal STIM1 antibody. B, representative recording showing that BAPTA‐AM‐evoked single channel activity in an inside‐out patch from a WT VSMC held at −80 mV was inhibited by bath application of a C‐terminal but not a N‐terminal STIM1 antibody. C, representative images from two different VSMCs treated with Triton, in which both N‐terminal and C‐terminal STIM1 antibodies WT identified translocation of STIM1 signalling (red) from the cytosol to the PM following treatment with BAPTA‐AM for 10 min. D, representative images from two different WT VSMCs not treated with Triton, in which N‐terminal, nor C‐terminal STIM1 antibodies identified STIM1 staining in unstimulated cells, and only the N‐terminal antibody revealed STIM1 staining at the PM following treatment with BAPTA‐AM.
Figure 11. Noradrenaline‐evoked TRPC1 channel activity is mediated by STIM1
A, bath application of noradrenaline evoked TRPC1 channel activity in a concentration‐dependent manner in cell‐attached patches from WT mesenteric artery VSMCs held at −80 mV, which were reduced in VSMCs expressing STIM1 shRNA1 and shRNA2 sequences compared to scrambled shRNA. B, mean data showing the inhibitory actions of STIM1 shRNA1 and shRNA2 on noradrenaline‐evoked TRPC1 channel activity (at least six patches in which every concentration of noradrenaline tested was cumulatively applied; ** P < 0.01, *** P < 0.001). C, PLA images from single WT VSMCs showing that application of noradrenaline for 5 min induced fluorescence signals (red), which indicated interactions between TRPC1 and STIM1, STIM1 and Gαq, and STIM1 and PLCβ1 predominantly at the PM.
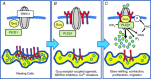
Figure 12. Proposed activation model of TRPC1‐based SOCs in contractile VSMCs
A, in resting VSMCs SR stores are filled with Ca2+, TRPC1‐based SOCs do not interact with Gαq, PLCβ1 or STIM1, and the channels are in a closed state. B, following store depletion of the SR, STIM1 proteins (red) are activated and translocated from the SR into the PM, where they interact with TRPC1. Following translocation, the N‐terminal EF hand of STIM1, which acts as a Ca2+ sensor within the SR, is exposed on the external surface of the cell, whereas the C‐terminal region is maintained within the cytosol. C, formation of store‐operated STIM1‐TRPC1 interactions enable the binding of Gαq and PLCβ1, which stimulate PLC activity, leading to PIP2 hydrolysis, formation of DAG, PKC stimulation, phosphorylation of TRPC1 subunits and channel opening.
Similar articles
- Evidence that Orai1 does not contribute to store-operated TRPC1 channels in vascular smooth muscle cells.
Shi J, Miralles F, Kinet JP, Birnbaumer L, Large WA, Albert AP. Shi J, et al. Channels (Austin). 2017 Jul 4;11(4):329-339. doi: 10.1080/19336950.2017.1303025. Epub 2017 Mar 16. Channels (Austin). 2017. PMID: 28301277 Free PMC article. - Store depletion induces Gαq-mediated PLCβ1 activity to stimulate TRPC1 channels in vascular smooth muscle cells.
Shi J, Miralles F, Birnbaumer L, Large WA, Albert AP. Shi J, et al. FASEB J. 2016 Feb;30(2):702-15. doi: 10.1096/fj.15-280271. Epub 2015 Oct 14. FASEB J. 2016. PMID: 26467792 Free PMC article. - Obligatory role for PKCδ in PIP2 -mediated activation of store-operated TRPC1 channels in vascular smooth muscle cells.
Martín-Aragón Baudel MAS, Shi J, Large WA, Albert AP. Martín-Aragón Baudel MAS, et al. J Physiol. 2020 Sep;598(18):3911-3925. doi: 10.1113/JP279947. Epub 2020 Jul 21. J Physiol. 2020. PMID: 32627185 Free PMC article. - Insights into Activation Mechanisms of Store-Operated TRPC1 Channels in Vascular Smooth Muscle.
Baudel MASM, Shi J, Large WA, Albert AP. Baudel MASM, et al. Cells. 2020 Jan 10;9(1):179. doi: 10.3390/cells9010179. Cells. 2020. PMID: 31936855 Free PMC article. Review. - The contribution of TRPC1 and STIM1 to capacitative Ca(2+) entry in pulmonary artery.
Ng LC, Airey JA, Hume JR. Ng LC, et al. Adv Exp Med Biol. 2010;661:123-35. doi: 10.1007/978-1-60761-500-2_8. Adv Exp Med Biol. 2010. PMID: 20204727 Review.
Cited by
- The deregulation of STIM1 and store operative calcium entry impaired aortic smooth muscle cells contractility in aortic medial degeneration.
Hong J, Hu Z, Wu Q, Tang C, Hu J, Chen R, Li B, Wang Z. Hong J, et al. Biosci Rep. 2019 Jan 3;39(1):BSR20181504. doi: 10.1042/BSR20181504. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30504131 Free PMC article. - Evidence that Orai1 does not contribute to store-operated TRPC1 channels in vascular smooth muscle cells.
Shi J, Miralles F, Kinet JP, Birnbaumer L, Large WA, Albert AP. Shi J, et al. Channels (Austin). 2017 Jul 4;11(4):329-339. doi: 10.1080/19336950.2017.1303025. Epub 2017 Mar 16. Channels (Austin). 2017. PMID: 28301277 Free PMC article. - Cross-Talk between Mechanosensitive Ion Channels and Calcium Regulatory Proteins in Cardiovascular Health and Disease.
Wang Y, Shi J, Tong X. Wang Y, et al. Int J Mol Sci. 2021 Aug 16;22(16):8782. doi: 10.3390/ijms22168782. Int J Mol Sci. 2021. PMID: 34445487 Free PMC article. Review. - MARCKS mediates vascular contractility through regulating interactions between voltage-gated Ca2+ channels and PIP2.
Jahan KS, Shi J, Greenberg HZE, Khavandi S, Baudel MM, Barrese V, Greenwood IA, Albert AP. Jahan KS, et al. Vascul Pharmacol. 2020 Sep;132:106776. doi: 10.1016/j.vph.2020.106776. Epub 2020 Jul 21. Vascul Pharmacol. 2020. PMID: 32707323 Free PMC article. - Integration of Ca2+ signaling regulates the breast tumor cell response to simvastatin and doxorubicin.
Abdoul-Azize S, Buquet C, Li H, Picquenot JM, Vannier JP. Abdoul-Azize S, et al. Oncogene. 2018 Sep;37(36):4979-4993. doi: 10.1038/s41388-018-0329-6. Epub 2018 May 23. Oncogene. 2018. PMID: 29795329
References
- Albert AP, Saleh SN & Large WA (2009). Identification of canonical transient receptor potential (TRPC) channel proteins in native vascular smooth muscle cells. Curr Med Chem 16, 1158–1165. - PubMed
- Albert AP (2011) Gating mechanisms of canonical transient receptor potential channel proteins: role of phosphoinositols and diacylglycerol. Adv Exp Med Biol 704, 391–411. - PubMed
- Alfonso S, Benito O, Alicia S, Angélica Z, Patricia G, Diana K & Vaca L (2008). Regulation of the cellular localization and function of human transient receptor potential channel 1 by other members of the TRPC family. Cell Calcium 43, 375–387. - PubMed
- Ambudkar IS, Ong HL, Liu X, Bandyopadhyay BC & Cheng KT (2007). TRPC1: functional link between functionally distinct different store‐operated calcium channels. Cell Calcium 42, 213–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 ES101684/ES/NIEHS NIH HHS/United States
- BB/J007226/1/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M018350/1/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous