Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy - PubMed (original) (raw)
Review
Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy
Jason J Rose et al. Am J Respir Crit Care Med. 2017.
Erratum in
- Erratum: Carbon Monoxide Poisoning: Pathogenesis, Management, and Future Directions of Therapy.
[No authors listed] [No authors listed] Am J Respir Crit Care Med. 2017 Aug 1;196(3):398-399. doi: 10.1164/rccm.1963erratum. Am J Respir Crit Care Med. 2017. PMID: 28762790 Free PMC article. No abstract available.
Abstract
Carbon monoxide (CO) poisoning affects 50,000 people a year in the United States. The clinical presentation runs a spectrum, ranging from headache and dizziness to coma and death, with a mortality rate ranging from 1 to 3%. A significant number of patients who survive CO poisoning suffer from long-term neurological and affective sequelae. The neurologic deficits do not necessarily correlate with blood CO levels but likely result from the pleiotropic effects of CO on cellular mitochondrial respiration, cellular energy utilization, inflammation, and free radical generation, especially in the brain and heart. Long-term neurocognitive deficits occur in 15-40% of patients, whereas approximately one-third of moderate to severely poisoned patients exhibit cardiac dysfunction, including arrhythmia, left ventricular systolic dysfunction, and myocardial infarction. Imaging studies reveal cerebral white matter hyperintensities, with delayed posthypoxic leukoencephalopathy or diffuse brain atrophy. Management of these patients requires the identification of accompanying drug ingestions, especially in the setting of intentional poisoning, fire-related toxic gas exposures, and inhalational injuries. Conventional therapy is limited to normobaric and hyperbaric oxygen, with no available antidotal therapy. Although hyperbaric oxygen significantly reduces the permanent neurological and affective effects of CO poisoning, a portion of survivors still have substantial morbidity. There has been some early success in therapies targeting the downstream inflammatory and oxidative effects of CO poisoning. New methods to directly target the toxic effect of CO, such as CO scavenging agents, are currently under development.
Keywords: carbon monoxide; carbon monoxide poisoning; mitochondria.
Figures
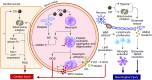
Figure 1.
Hemoglobin (Hb) and mitochondrial effects of CO. Normal: Hb binds oxygen and delivers it to peripheral tissue with low PO2. Reduced cytochrome c (Cytc) transfers its electron (e−) to cytochrome c oxidase (COX) subunit 1 (CytA: binuclear center with heme a and copper [CuA]). The electron reduces oxygen (O2) at subunit 2 (CytA3: binuclear center with heme a3 and copper [CuB]), forming water and transporting a proton (H+) through the inner mitochondrial membrane. CO toxicity: CO competitively binds to Hb with O2, reducing total oxygen carrying capacity by: (1) preferentially binding to CO instead of O2 (anemia-like effect); and (2) stabilizing the relaxed quaternary state of Hb, which binds to O2 with higher affinity and will not release it in low PO2 environment. CO binds competitively with O2 at the reduced heme a3 in subunit 2. This causes: (1) inhibition of the reduction of O2 to water (the end destination of electrons in the electron transport chain); (2) cessation of the transfer of H+ into the intermembrane space, shutting down ATP generation through ATP synthase; and (3) accumulation of electrons entering the electron transport chain through complexes I and III, which can produce superoxide, leading to deleterious effects. ETC = electron transport chain.
Figure 2.
Inflammatory mechanisms of CO toxicity. CO activates platelets by displacing platelet nitric oxide (NO) from surface hemoproteins. NO reacts with oxygen free radicals (O2−) to produce peroxynitrite (ONOO−), which inhibits mitochondrial function and activates platelets and neutrophils itself. Inhibition of mitochondria leads to further production of reactive oxygen species (ROS) and causes release of free heme and ensuing increase of heme oxygenase (HO)-1, further causing oxidative stress. HO-1 metabolizes free heme to produce more endogenous CO, creating a positive-feedback loop locally. Activated neutrophils will degranulate and release myeloperoxidase (MPO), causing more neutrophil activation, as well as adhesion. Proteases released from neutrophils can oxidize endothelial cell xanthine dehydrogenase (XD) to xanthine oxidase (XO), generating ROS, causing cellular damage as well as lipid peroxidation, specifically on myelin basic protein (MBP). When peroxidated, MBP forms adducts that cause lymphocyte proliferation, microglia activation, and, ultimately, neurologic injury. The general effects of hypoxia and the effect of CO toxicity directly on mitochondria cause glutamate release, which activates _N_-methyl-
d
-aspartate (NMDA) receptors, further leading to neurologic injury.
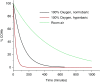
Figure 3.
Decay of the carboxyhemoglobin (COHb) species under therapeutic treatments. Half-life values of COHb in room air (320 min), 100% normobaric oxygen (74 min), and 100% hyperbaric oxygen (HBO2; 20 min) determined from Refs. , , , –.
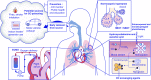
Figure 4.
Current and future therapeutic targets of CO poisoning. Prevention and early removal from the CO-poisoned environments are tenants to current management. Hyperbaric oxygen therapy (HBOT) and normobaric oxygen therapy (NBOT) increase the partial pressure of oxygen in the alveoli, increasing the rate of CO dissociation from hemoglobin (Hb; _see_Figure 3). Normocapnic hyperpnea increases ventilation through delivery of CO2 in addition to increasing the partial pressure of O2. Hydroxycobalamine and ascorbic acid increase the rate of CO conversion to carbon dioxide (CO2). Extracorporeal membrane oxygenation (ECMO) supports blood pressure and gas exchange, delivering oxygen even in the setting of acute respiratory distress syndrome from inhalational injury. Phototherapy increases the dissociation of CO from Hb in the blood stream. Finally, CO-scavenging agents, such as porphyrine complexes or modified globin proteins, bind to CO from cellular heme proteins, and act as a “sink,” removing CO from the body when excreted.
Comment in
- Reply: Better Studies Are Needed to Guide Treatment of Carbon Monoxide Poisoning.
Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Rose JJ, et al. Am J Respir Crit Care Med. 2017 Mar 1;195(5):694-695. doi: 10.1164/rccm.201612-2463LE. Am J Respir Crit Care Med. 2017. PMID: 28248143 Free PMC article. No abstract available. - Better Studies Are Needed to Guide Treatment of Carbon Monoxide Poisoning.
Juurlink DN, Buckley NA, Eddleston M. Juurlink DN, et al. Am J Respir Crit Care Med. 2017 Mar 1;195(5):694. doi: 10.1164/rccm.201611-2255LE. Am J Respir Crit Care Med. 2017. PMID: 28248145 No abstract available. - Carbon Monoxide Exposure in Workplaces, Including Coffee Processing Facilities.
Hawley B, Cox-Ganser JM, Cummings KJ. Hawley B, et al. Am J Respir Crit Care Med. 2017 Oct 15;196(8):1080-1081. doi: 10.1164/rccm.201703-0513LE. Am J Respir Crit Care Med. 2017. PMID: 28471692 Free PMC article. No abstract available. - Reply: Carbon Monoxide Exposure in Workplaces, Including Coffee Processing Facilities.
Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva S, Tejero J, Gladwin MT. Rose JJ, et al. Am J Respir Crit Care Med. 2017 Oct 15;196(8):1081-1082. doi: 10.1164/rccm.201704-0773LE. Am J Respir Crit Care Med. 2017. PMID: 28471722 Free PMC article. No abstract available.
Similar articles
- Carbon monoxide poisoning.
Weaver LK. Weaver LK. Undersea Hyperb Med. 2020 First Quarter;47(1):151-169. doi: 10.22462/01.03.2020.17. Undersea Hyperb Med. 2020. PMID: 32176957 Review. - Neurocognitive sequelae after carbon monoxide poisoning and hyperbaric oxygen therapy.
Ning K, Zhou YY, Zhang N, Sun XJ, Liu WW, Han CH. Ning K, et al. Med Gas Res. 2020 Jan-Mar;10(1):30-36. doi: 10.4103/2045-9912.279981. Med Gas Res. 2020. PMID: 32189667 Free PMC article. Review. - Acute carbon monoxide poisoning in a regional hospital in Hong Kong: historical cohort study.
Chan MY, Au TTs, Leung KS, Yan WW. Chan MY, et al. Hong Kong Med J. 2016 Feb;22(1):46-55. doi: 10.12809/hkmj144529. Epub 2016 Jan 15. Hong Kong Med J. 2016. PMID: 26769825 - Clinical Outcomes and Mortality Impact of Hyperbaric Oxygen Therapy in Patients With Carbon Monoxide Poisoning.
Rose JJ, Nouraie M, Gauthier MC, Pizon AF, Saul MI, Donahoe MP, Gladwin MT. Rose JJ, et al. Crit Care Med. 2018 Jul;46(7):e649-e655. doi: 10.1097/CCM.0000000000003135. Crit Care Med. 2018. PMID: 29629990 Free PMC article. - Hyperbaric oxygen therapy for carbon monoxide poisoning.
Weaver LK. Weaver LK. Undersea Hyperb Med. 2014 Jul-Aug;41(4):339-54. Undersea Hyperb Med. 2014. PMID: 25109087 Review.
Cited by
- Association of carbon monoxide poisoning with cardiovascular disease risk: A systematic review and meta-analysis.
Du W, Tian Z, Lv B, Wang P, Wang H, Ding S, Tian Z, Zhou J, Jiao W, Zhang X, Gao H. Du W, et al. Heliyon. 2024 Jul 14;10(14):e34062. doi: 10.1016/j.heliyon.2024.e34062. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39113966 Free PMC article. - The Cannabinoid WIN 55,212-2 Reduces Delayed Neurologic Sequelae After Carbon Monoxide Poisoning by Promoting Microglial M2 Polarization Through ST2 Signaling.
Du JJ, Liu ZQ, Yan Y, Xiong J, Jia XT, Di ZL, Ren JJ. Du JJ, et al. J Mol Neurosci. 2020 Mar;70(3):422-432. doi: 10.1007/s12031-019-01429-2. Epub 2019 Nov 15. J Mol Neurosci. 2020. PMID: 31732924 - Prognostic factors of hyperbaric oxygen therapy for patients with delayed encephalopathy after acute carbon monoxide poisoning.
Huang F, Yang L, Tan Z, Yang B, Liu P, Li Z, Shi W, Peng K, Yuan J, He Q, Yang L, Li X, Li C, Chen D, Peng Z. Huang F, et al. Heliyon. 2022 Dec 15;8(12):e12351. doi: 10.1016/j.heliyon.2022.e12351. eCollection 2022 Dec. Heliyon. 2022. PMID: 36582705 Free PMC article. - Oxygenation Performance of Different Non-Invasive Devices for Treatment of Decompression Illness and Carbon Monoxide Poisoning.
Köhler A, Zoll FM, Ploner T, Hammer A, Joannidis M, Tilg H, Finkenstedt A, Hartig F. Köhler A, et al. Front Physiol. 2022 Apr 26;13:885898. doi: 10.3389/fphys.2022.885898. eCollection 2022. Front Physiol. 2022. PMID: 35557974 Free PMC article. - A Comparison of Occupational CO Levels, HbCO, and Lung Functions Between Grill and Non-grill Street Vendors.
Soeroso NN, Intan TK, Jery, Ananda FR. Soeroso NN, et al. Med Arch. 2021 Aug;75(4):286-290. doi: 10.5455/medarh.2021.75.286-290. Med Arch. 2021. PMID: 34759449 Free PMC article.
References
- Hampson NB. Emergency department visits for carbon monoxide poisoning in the Pacific Northwest. 1998;16:695–698. - PubMed
- Hampson NB, Weaver LK. Carbon monoxide poisoning: a new incidence for an old disease. 2007;34:163–168. - PubMed
- Centers for Disease Control and Prevention (CDC) Carbon monoxide—related deaths—United States, 1999–2004. 2007;56:1309–1312. - PubMed
- Hampson NB. U.S. mortality due to carbon monoxide poisoning, 1999-2014: accidental and intentional deaths. 2016;13:1768–1774. - PubMed
- Hampson NB, Bodwin D. Toxic CO-ingestions in intentional carbon monoxide poisoning. 2013;44:625–630. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical