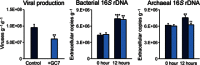Virus-mediated archaeal hecatomb in the deep seafloor - PubMed (original) (raw)
Virus-mediated archaeal hecatomb in the deep seafloor
Roberto Danovaro et al. Sci Adv. 2016.
Abstract
Viruses are the most abundant biological entities in the world's oceans, and they play a crucial role in global biogeochemical cycles. In deep-sea ecosystems, archaea and bacteria drive major nutrient cycles, and viruses are largely responsible for their mortality, thereby exerting important controls on microbial dynamics. However, the relative impact of viruses on archaea compared to bacteria is unknown, limiting our understanding of the factors controlling the functioning of marine systems at a global scale. We evaluate the selectivity of viral infections by using several independent approaches, including an innovative molecular method based on the quantification of archaeal versus bacterial genes released by viral lysis. We provide evidence that, in all oceanic surface sediments (from 1000- to 10,000-m water depth), the impact of viral infection is higher on archaea than on bacteria. We also found that, within deep-sea benthic archaea, the impact of viruses was mainly directed at members of specific clades of Marine Group I Thaumarchaeota. Although archaea represent, on average, ~12% of the total cell abundance in the top 50 cm of sediment, virus-induced lysis of archaea accounts for up to one-third of the total microbial biomass killed, resulting in the release of ~0.3 to 0.5 gigatons of carbon per year globally. Our results indicate that viral infection represents a key mechanism controlling the turnover of archaea in surface deep-sea sediments. We conclude that interactions between archaea and their viruses might play a profound, previously underestimated role in the functioning of deep-sea ecosystems and in global biogeochemical cycles.
Keywords: Deep-sea ecosystems; archaea; bacteria; biodiversity; ecosystem functioning; microbial loop; virus-host interactions.
Figures
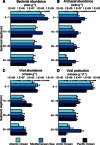
Fig. 1. Bacteria, archaea, and viruses in deep-sea sediments.
Reported are bacterial (A) and archaeal (B) abundance obtained by catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH), and viral abundance (C) and production (D)along the vertical profiles at depth intervals of 0 to 1, 10 to 15, 20 to 30, and 40 to 50 cm in deep-sea sediments collected in the Arctic Ocean, Atlantic Ocean, and Pacific Ocean and Mediterranean Sea, with average values and SDs.
Fig. 2. Archaeal viruses in the viromes.
Relative contribution (expressed as percentage) of the sequences belonging to each archaeal virotype to the total number of sequences of archaeal viruses identified in each virome from the different sampling sites.
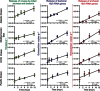
Fig. 3. Release of bacterial and archaeal genes due to viral lysis.
The panel shows the release of viruses from killed archaea and bacteria (left column) and consequent shift of the 16_S_ rRNA genes from bacteria (central column) and archaea (right column) to the extracellular DNA pools during time-course experiments conducted on deep-sea sediments from the Atlantic Ocean (first row), Mediterranean Sea (second row), Arctic Ocean (third row), and Pacific Ocean (bottom row). Reported are means ± SDs at the different time intervals, as well as the equation and _R_2 value of each line of best fit.
Fig. 4. Effect of the inhibition of archaeal and bacterial metabolism.
Reported are, from left to right, viral abundance, bacterial 16_S_ rRNA gene copies in the extracellular DNA, and archaeal 16_S_ rRNA gene copies in the extracellular DNA, during time-course incubations of untreated (control, blue bars) and treated (light blue bars, following addition of GC7 plus antibiotics to inhibit archaeal and bacterial metabolism) deep-sea sediment samples. Reported are average values and SDs. Asterisks indicate the significant increase (**P < 0.01) in the abundance of viruses and the number of bacterial or archaeal 16_S_ rRNA gene copies after 12 hours of incubation in untreated (control) samples. ns, no significant differences occurring over time in treated samples; rDNA, ribosomal DNA.
Fig. 5. Effect of the selective inhibition of archaeal metabolism.
Reported are, from left to right, viral production, bacterial 16_S_ rRNA gene copies in the extracellular DNA, and archaeal 16_S_ rRNA gene copies in the extracellular DNA, during time-course incubations of untreated (controls, blue bars) and treated (light blue bars, following addition of GC7 to selectively inhibit archaeal metabolism) deep-sea sediment samples. Reported are average values and SDs. Asterisks (**P < 0.01; ***P < 0.001) indicate a significant decrease after 12 hours of incubation for viral production rates in treated samples, a significant increase in the number of both bacterial and archaeal 16_S_ rRNA gene copies in control samples, or a significant increase in the number of bacterial only 16_S_ rRNA gene copies in treated samples. ns, no significant increase in archaeal 16_S_ rRNA gene copies occurring over time in treated samples.
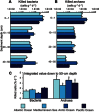
Fig. 6. Virus-induced mortality of bacteria and archaea.
Reported are average values and SDs of the virus-induced mortality of bacteria (A) and archaea (B) along vertical profiles at depth intervals of 0 to 1, 10 to 15, 20 to 30, and 40 to 50 cm, and the integrated values for the top 50 cm (C), of deep-sea sediments collected from Arctic Ocean, Atlantic Ocean, and Pacific Ocean and Mediterranean Sea. Asterisks indicate significantly higher (*P < 0.05) virus-induced mortality rates of archaea as found for all the oceanographic regions investigated.
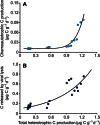
Fig. 7. Relationships between different microbial processes occurring in surface deep-sea sediments.
Reported are the relationship between total heterotrophic C production and (A) chemoautotrophic C production in surface deep-sea sediments (equations of the line of best fit, y = 0.011 + 0.019_x_7.67; _R_2 = 0.918; n = 18; P < 0.01) and (B) the amount of C released by viral lysis of host cells (equations of the line of best fit, y = 0.0988e1.58x; _R_2 = 0.831; n = 18; P < 0.01).
Similar articles
- Potential impact of global climate change on benthic deep-sea microbes.
Danovaro R, Corinaldesi C, Dell'Anno A, Rastelli E. Danovaro R, et al. FEMS Microbiol Lett. 2017 Dec 15;364(23). doi: 10.1093/femsle/fnx214. FEMS Microbiol Lett. 2017. PMID: 29045616 Review. - Macroecological drivers of archaea and bacteria in benthic deep-sea ecosystems.
Danovaro R, Molari M, Corinaldesi C, Dell'Anno A. Danovaro R, et al. Sci Adv. 2016 Apr 29;2(4):e1500961. doi: 10.1126/sciadv.1500961. eCollection 2016 Apr. Sci Adv. 2016. PMID: 27386507 Free PMC article. - Major viral impact on the functioning of benthic deep-sea ecosystems.
Danovaro R, Dell'Anno A, Corinaldesi C, Magagnini M, Noble R, Tamburini C, Weinbauer M. Danovaro R, et al. Nature. 2008 Aug 28;454(7208):1084-7. doi: 10.1038/nature07268. Nature. 2008. PMID: 18756250 - Virus decomposition provides an important contribution to benthic deep-sea ecosystem functioning.
Dell'Anno A, Corinaldesi C, Danovaro R. Dell'Anno A, et al. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):E2014-9. doi: 10.1073/pnas.1422234112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25848024 Free PMC article. - Viruses of archaea: Structural, functional, environmental and evolutionary genomics.
Krupovic M, Cvirkaite-Krupovic V, Iranzo J, Prangishvili D, Koonin EV. Krupovic M, et al. Virus Res. 2018 Jan 15;244:181-193. doi: 10.1016/j.virusres.2017.11.025. Epub 2017 Nov 22. Virus Res. 2018. PMID: 29175107 Free PMC article. Review.
Cited by
- A virus-borne DNA damage signaling pathway controls the lysogeny-induction switch in a group of temperate pleolipoviruses.
Chen Z, Liu Y, Wang Y, Du X, Deng X, Xiang J, Wang Y, Wang J, Krupovic M, Du S, Chen X. Chen Z, et al. Nucleic Acids Res. 2023 Apr 24;51(7):3270-3287. doi: 10.1093/nar/gkad125. Nucleic Acids Res. 2023. PMID: 36864746 Free PMC article. - Novel Viral Communities Potentially Assisting in Carbon, Nitrogen, and Sulfur Metabolism in the Upper Slope Sediments of Mariana Trench.
Zhao J, Jing H, Wang Z, Wang L, Jian H, Zhang R, Xiao X, Chen F, Jiao N, Zhang Y. Zhao J, et al. mSystems. 2022 Feb 22;7(1):e0135821. doi: 10.1128/msystems.01358-21. Epub 2022 Jan 4. mSystems. 2022. PMID: 35089086 Free PMC article. - Ammonia-oxidizing archaea have similar power requirements in diverse marine oxic sediments.
Zhao R, Mogollón JM, Roerdink DL, Thorseth IH, Økland I, Jørgensen SL. Zhao R, et al. ISME J. 2021 Dec;15(12):3657-3667. doi: 10.1038/s41396-021-01041-6. Epub 2021 Jun 22. ISME J. 2021. PMID: 34158628 Free PMC article. - The enigmatic archaeal virosphere.
Prangishvili D, Bamford DH, Forterre P, Iranzo J, Koonin EV, Krupovic M. Prangishvili D, et al. Nat Rev Microbiol. 2017 Nov 10;15(12):724-739. doi: 10.1038/nrmicro.2017.125. Nat Rev Microbiol. 2017. PMID: 29123227 Review. - Viruses in Extreme Environments, Current Overview, and Biotechnological Potential.
Gil JF, Mesa V, Estrada-Ortiz N, Lopez-Obando M, Gómez A, Plácido J. Gil JF, et al. Viruses. 2021 Jan 8;13(1):81. doi: 10.3390/v13010081. Viruses. 2021. PMID: 33430116 Free PMC article. Review.
References
- Fuhrman J. A., Marine viruses and their biogeochemical and ecological effects. Nature 399, 541–548 (1999). - PubMed
- Weinbauer M. G., Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 28, 127–181 (2004). - PubMed
- Suttle C. A., Review viruses in the sea. Nature 437, 356–361 (2005). - PubMed
- Suttle C. A., Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 5, 801–812 (2007). - PubMed
- Danovaro R., Dell’Anno A., Corinaldesi C., Magagnini M., Noble R., Tamburini C., Weinbauer M., Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 454, 1084–1087 (2008). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources