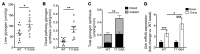Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance - PubMed (original) (raw)
. 2016 Nov 1;126(11):4361-4371.
doi: 10.1172/JCI86013. Epub 2016 Oct 17.
Anila K Madiraju, Brandon M Gassaway, Michael Marcel, Ali R Nasiri, Gina Butrico, Melissa J Marcucci, Dongyan Zhang, Abudukadier Abulizi, Xian-Man Zhang, William Philbrick, Stevan R Hubbard, Michael J Jurczak, Varman T Samuel, Jesse Rinehart, Gerald I Shulman
- PMID: 27760050
- PMCID: PMC5096902
- DOI: 10.1172/JCI86013
Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance
Max C Petersen et al. J Clin Invest. 2016.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a risk factor for type 2 diabetes (T2D), but whether NAFLD plays a causal role in the pathogenesis of T2D is uncertain. One proposed mechanism linking NAFLD to hepatic insulin resistance involves diacylglycerol-mediated (DAG-mediated) activation of protein kinase C-ε (PKCε) and the consequent inhibition of insulin receptor (INSR) kinase activity. However, the molecular mechanism underlying PKCε inhibition of INSR kinase activity is unknown. Here, we used mass spectrometry to identify the phosphorylation site Thr1160 as a PKCε substrate in the functionally critical INSR kinase activation loop. We hypothesized that Thr1160 phosphorylation impairs INSR kinase activity by destabilizing the active configuration of the INSR kinase, and our results confirmed this prediction by demonstrating severely impaired INSR kinase activity in phosphomimetic T1160E mutants. Conversely, the INSR T1160A mutant was not inhibited by PKCε in vitro. Furthermore, mice with a threonine-to-alanine mutation at the homologous residue Thr1150 (InsrT1150A mice) were protected from high fat diet-induced hepatic insulin resistance. InsrT1150A mice also displayed increased insulin signaling, suppression of hepatic glucose production, and increased hepatic glycogen synthesis compared with WT controls during hyperinsulinemic clamp studies. These data reveal a critical pathophysiological role for INSR Thr1160 phosphorylation and provide further mechanistic links between PKCε and INSR in mediating NAFLD-induced hepatic insulin resistance.
Figures
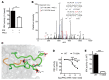
Figure 1. PKCε phosphorylates the INSR at Thr1160.
(A) IRK activity with and without equimolar PKCε preincubation in vitro. (B) Overlaid MS/MS spectra of the T1160-phosphorylated peptide DIYETPDYYRK (identified only in IRK + PKCε samples; unique peaks are shown in blue) and the Y1162-phosphorylated peptide DIYETDYPYRK (identified in samples with IRK alone; unique peaks are shown in red). An Andromeda score of 167.67, a localization probability of 68%, and a 4.3 Delta score between pT1160 assignment and pY1162 assignment supported assignment of phosphorylation to T1160. pT, phosphothreonine. pY, phosphotyrosine. Y and B series MS/MS fragment ions are defined with respect to primary amino acid sequence at upper right. (C) Mechanism by which Thr1160 phosphorylation inhibits IRK activity. The crystal structure of phosphorylated, activated IRK (19) is shown, with the activation loop in green, the catalytic loop in orange, and the rest of IRK in gray (surface representation). The 3 phosphotyrosines in the activation loop are shown as sticks, as is Thr1160 (carbon atoms in green, oxygen atoms in red, nitrogen atoms in blue, and phosphorus atoms in black). The side chain of (unphosphorylated) Thr1160 is hydrogen bonded (black dashed line) to the backbone carbonyl oxygen of phosphorylated Tyr1162 (pTyr1162). If Thr1160 were phosphorylated (hypothetical phosphate group shown semitransparently), the activation loop could not adopt this configuration because of steric clashes and electrostatic repulsion with pTyr1162. (D) In vitro kinase activity of recombinant WT or T1160A IRK at several PKCε concentrations. (E) In vitro kinase activity of recombinant WT or T1160E IRK. Data represent the mean ± SEM. (A) *P < 0.05, by unpaired, 2-tailed Student’s t test for 5 technical replicates per group; (D and E) ***P < 0.0005, by unpaired, 2-tailed Student’s t test for 3 to 4 technical replicates per group.
Figure 2. INSR T1160 phosphorylation impairs insulin signaling.
(A) Autophosphorylation of WT or T1160E INSR in transfected HeLa cells treated for 15 minutes with 100 nM insulin. (B) In vitro kinase activity of GFP-tagged WT or T1160E INSR immunoprecipitated from transfected HeLa cells treated for 10 minutes with 100 nM insulin. (C) MS/MS fragmentation spectrum identifying Thr1160 phosphorylation in cells. McArdle rat hepatoma cells were transfected with WT GFP-tagged INSR. GFP immunoprecipitates were run on SDS-PAGE gels, and INSR-GFP gel bands were excised, digested with trypsin, enriched for phosphopeptides with TiO2, and analyzed by LC-MS/MS. Peptides were identified using MaxQuant software, and phosphosite assignments are shown by color coding. Localization probabilities for the 3 identified peptides are: DIY(1)ETDYYR; DIYET(.785)DYYR; and DIYETDY(.364)Y(.574)R. The Andromeda scores were as follows: pTyr1158 = 91.867; Thr1160 = 52.571; and pTyr1162 = 105.52. These scores support the coexistence of multiple singly phosphorylated species (i.e., pTyr1158, pThr1160, pTyr1162, and pTyr1163) in the biological sample. Data represent the mean ± SEM. (B) *P < 0.05 and ***P < 0.0005, by 2-way ANOVA. n = 2 (basal) and n = 4 (insulin) biological replicates per group. IB, immunoblot.
Figure 3. InsrT1150A mice are protected from HFD-induced hepatic insulin resistance.
Male InsrT1150A and WT littermate mice were fed an HFD for 8 to 10 days and fasted overnight before hyperinsulinemic-euglycemic clamp studies. (A) Glucose infusion rate. (B) Mean steady-state glucose infusion rate. (C) EGP during the basal and the steady-state periods of the clamp. (D) Insulin suppression of EGP during the clamp. (E and F) Phosphorylation of INSR, AKT, and GSK3β in overnight-fasted (– insulin, basal) or post-clamp (+ insulin, clamp) mice. (G) Immunoprecipitable liver IRK activity in post-clamp mice. *P < 0.05 and **P < 0.005, by unpaired, 2-tailed Student’s t test. Data represent the mean ± SEM. n = 6 mice per group.
Figure 4. InsrT1150A mice fed a chronic HFD are protected from hepatic, but not skeletal muscle, insulin resistance.
Male InsrT1150A and WT littermate mice were fed an HFD for 6 weeks and fasted overnight before hyperinsulinemic-euglycemic clamp studies. (A) Plasma glucose and glucose infusion rates during the clamp. (B) Mean steady-state glucose infusion rate. (C) EGP during the basal and steady-state periods of the clamp. (D) Suppression of EGP during the clamp, expressed as the percentage of basal EGP. (E and F) Hepatic AKT phosphorylation in post-clamp mice. (G) Whole-body glucose uptake during the steady-state period of the clamp. (H) 14C-2-deoxyglucose uptake during the clamp in gastrocnemius/soleus muscle. (A–H) Data represent the mean ± SEM . n = 5–7 mice per group. *P < 0.05, by unpaired, 2-tailed Student’s t test.
Figure 5. Increased insulin-stimulated hepatic glycogen synthesis in HFD-fed InsrT1150A mice.
Male InsrT1150A and WT littermate mice were fed an HFD for 8 to 10 days and fasted for 13 hours before undergoing hyperinsulinemic-hyperglycemic clamp studies. [U-13C]-glucose was infused to assess glycogen synthesis rates. (A) Total liver glycogen content after clamp studies. (B) Glycogen synthesis flux through the direct pathway during the clamp. (C) Total glycogen synthesis flux through both direct and indirect pathways during the clamp. (D) Relative Gck mRNA expression in fasted and post-clamp mice measured by qPCR and normalized to basal WT expression. (A–C) Data represent the mean ± SEM. n = 9 WT and n = 10 InsrT1150A mice per group. *P < 0.05 and **P < 0.005, by unpaired, 2-tailed Student’s t test. (D) Data represent the mean ± SEM. n = 4 (basal) and n = 5–7 (post-clamp) mice per group. *P < 0.05 and ***P < 0.0005, by 2-way ANOVA with Holm-Sidak correction for multiple comparisons.
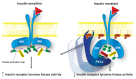
Figure 6. Proposed mechanism of lipid-induced hepatic insulin resistance.
In normal, insulin-sensitive liver (left), Thr1160 does not interfere with IRK activation, and downstream signaling proceeds normally upon insulin binding. In NAFLD (right), DAG accumulation promotes membrane translocation of PKCε, which in turn phosphorylates INSR Thr1160 to impair IRK activity and thereby induces hepatic insulin resistance. Y, tyrosine; P, phosphate.
Similar articles
- Short-term overnutrition induces white adipose tissue insulin resistance through sn-1,2-diacylglycerol/PKCε/insulin receptor Thr1160 phosphorylation.
Lyu K, Zhang D, Song J, Li X, Perry RJ, Samuel VT, Shulman GI. Lyu K, et al. JCI Insight. 2021 Feb 22;6(4):e139946. doi: 10.1172/jci.insight.139946. JCI Insight. 2021. PMID: 33411692 Free PMC article. - PKCε contributes to lipid-induced insulin resistance through cross talk with p70S6K and through previously unknown regulators of insulin signaling.
Gassaway BM, Petersen MC, Surovtseva YV, Barber KW, Sheetz JB, Aerni HR, Merkel JS, Samuel VT, Shulman GI, Rinehart J. Gassaway BM, et al. Proc Natl Acad Sci U S A. 2018 Sep 18;115(38):E8996-E9005. doi: 10.1073/pnas.1804379115. Epub 2018 Sep 4. Proc Natl Acad Sci U S A. 2018. PMID: 30181290 Free PMC article. - Ceramide synthesis inhibitors prevent lipid-induced insulin resistance through the DAG-PKCε-insulin receptorT1150 phosphorylation pathway.
Xu W, Zhang D, Ma Y, Gaspar RC, Kahn M, Nasiri A, Murray S, Samuel VT, Shulman GI. Xu W, et al. Cell Rep. 2024 Oct 22;43(10):114746. doi: 10.1016/j.celrep.2024.114746. Epub 2024 Sep 19. Cell Rep. 2024. PMID: 39302831 - Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models.
Shafrir E, Ziv E, Mosthaf L. Shafrir E, et al. Ann N Y Acad Sci. 1999 Nov 18;892:223-46. doi: 10.1111/j.1749-6632.1999.tb07798.x. Ann N Y Acad Sci. 1999. PMID: 10842665 Review.
Cited by
- Effects of Fish Oil and Grape Seed Extract Combination on Hepatic Endogenous Antioxidants and Bioactive Lipids in Diet-Induced Early Stages of Insulin Resistance in Rats.
Taltavull N, Miralles-Pérez B, Nogués MR, Ramos-Romero S, Méndez L, Medina I, Torres JL, Romeu M. Taltavull N, et al. Mar Drugs. 2020 Jun 16;18(6):318. doi: 10.3390/md18060318. Mar Drugs. 2020. PMID: 32560216 Free PMC article. - Adipose-derived extracellular vesicles - a novel cross-talk mechanism in insulin resistance, non-alcoholic fatty liver disease, and polycystic ovary syndrome.
Mladenović D, Vesković M, Šutulović N, Hrnčić D, Stanojlović O, Radić L, Macut JB, Macut D. Mladenović D, et al. Endocrine. 2024 Jul;85(1):18-34. doi: 10.1007/s12020-024-03702-w. Epub 2024 Jan 29. Endocrine. 2024. PMID: 38285412 Review. - Hepatic insulin sensitivity is improved in high-fat diet-fed Park2 knockout mice in association with increased hepatic AMPK activation and reduced steatosis.
Edmunds LR, Huckestein BR, Kahn M, Zhang D, Chu Y, Zhang Y, Wendell SG, Shulman GI, Jurczak MJ. Edmunds LR, et al. Physiol Rep. 2019 Nov;7(21):e14281. doi: 10.14814/phy2.14281. Physiol Rep. 2019. PMID: 31724300 Free PMC article. - Sex- and strain-specific effects of mitochondrial uncoupling on age-related metabolic diseases in high-fat diet-fed mice.
Goedeke L, Murt KN, Di Francesco A, Camporez JP, Nasiri AR, Wang Y, Zhang XM, Cline GW, de Cabo R, Shulman GI. Goedeke L, et al. Aging Cell. 2022 Feb;21(2):e13539. doi: 10.1111/acel.13539. Epub 2022 Jan 28. Aging Cell. 2022. PMID: 35088525 Free PMC article. - Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications.
Sangwung P, Petersen KF, Shulman GI, Knowles JW. Sangwung P, et al. Endocrinology. 2020 Apr 1;161(4):bqaa017. doi: 10.1210/endocr/bqaa017. Endocrinology. 2020. PMID: 32060542 Free PMC article. Review.
References
- Silverman JF, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85(10):1349–1355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 DK099402/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- T32 GM007205/GM/NIGMS NIH HHS/United States
- F30 DK104596/DK/NIDDK NIH HHS/United States
- KL2 TR001862/TR/NCATS NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous