Epidermal grafting for wound healing: a review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing - PubMed (original) (raw)
Review
Epidermal grafting for wound healing: a review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing
Muholan Kanapathy et al. Int Wound J. 2017 Feb.
Abstract
Epidermal grafting for wound healing involves the transfer of the epidermis from a healthy location to cover a wound. The structural difference of the epidermal graft in comparison to the split-thickness skin graft and full-thickness skin graft contributes to the mechanism of effect. While skin grafting is an epidermal transfer, little is known about the precise mechanism of wound healing by epidermal graft. This paper aims to explore the evolution of the epidermal graft harvesting system over the last five decades, the structural advantages of epidermal graft for wound healing and the current hypotheses on the mechanism of wound healing by epidermal graft. Three mechanisms are proposed: keratinocyte activation, growth factor secretion and reepithelialisation from the wound edge. We evaluate and explain how these processes work and integrate to promote wound healing based on the current in vivo and in vitro evidence. We also review the ongoing clinical trials evaluating the efficacy of epidermal graft for wound healing. The epidermal graft is a promising alternative to the more invasive conventional surgical techniques as it is simple, less expensive and reduces the surgical burden for patients in need of wound coverage.
Keywords: Epidermal graft; Healing mechanism; Skin graft; Ultrastructure; Wound healing.
© 2016 Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures
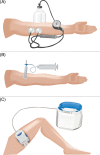
Figure 1
Epidermal graft harvesting systems. (A) The Dermovac system, which consists of a pair of transparent plexiglass suction cups and a handheld pump. (B) The syringe system, which consists of a small syringe with the piston removed and connected to a larger syringe through a three‐way connector. The three‐way connector is locked to maintain the negative pressure throughout the procedure. (C) The
CelluTome
Epidermal Harvesting System, which consists of a control unit connected to a vacuum head.
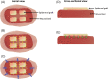
Figure 2
Ultrastructure of dermal–epidermal junction (
DEJ
) and blister cavity. The
DEJ
consists of four zones: membrane of the basal keratinocytes, lamina lucida, lamina densa and sub‐basal lamina. Hemi‐desmosomes, present at the dermal pole of the basal keratinocytes, link to anchoring filaments that connect the basal keratinocytes to the lamina lucida. Achoring fibrils link the lamina densa and the dermal matrix. Continuous negative pressure forms a blister at the level of sub‐basal lamina.
Figure 3
Mechanism of healing by epidermal graft (
EG
). (A–C) The aerial view of four
EGs
on a healthy wound bed. (D,E) The cross‐sectional view of an
EG
on a wound bed. Upon grafting (B), the keratinocytes within the
EG
are activated and migrate onto the wound bed (yellow arrows resembles keratinocyte migration). The activated keratinocytes concurrently secrete growth factors to the wound bed to stimulate the endogenous process of wound healing (E) (green arrows resemble growth factor expression). The activated keratinocytes and the growth factors stimulate the wound edge keratinocytes to migrate into the wound, accelerating reepithelialisation from the wound edge (C) (blue arrows resemble the migration of the wound edge keratinocyte into the wound).
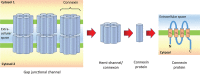
Figure 4
The structural organisation of the gap junctional protein, the Connexin. Each Connexin is made of a paired hemi‐channel known as a Connexon, which consists of six Connexin protein subunits. Each Connexin protein subunit has four alpha‐helical transmembrane proteins, two extracellular loops, a cytoplasmic loop and a N‐ and C‐terminus located within the cytoplasm 52. The C‐terminus binds to cytoskeletal elements within the cells to regulate cellular migratory properties 52.
References
- Hachach‐Haram N, Bystrzonowski N, Kanapathy M, Edmondson SJ, Twyman L, Richards T, Mosahebi A. The use of epidermal grafting for the management of acute wounds in the outpatient setting. J Plast Reconstr Aesthet Surg 2015;68:1317–8. - PubMed
- Richmond NA, Lamel SA, Braun LR, Vivas AC, Serena T, Kirsner RS. Epidermal grafting using a novel suction blister‐harvesting system for the treatment of pyoderma gangrenosum. JAMA Dermatol 2014;150:999–1000. - PubMed
- Serena T, Francius A, Taylor C, MacDonald J. Use of a novel epidermal harvesting system in resource‐poor countries. Adv Skin Wound Care 2015;28:107–12. - PubMed
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 1975;6:331–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical