A Cinnamon-Derived Procyanidin Compound Displays Anti-HIV-1 Activity by Blocking Heparan Sulfate- and Co-Receptor- Binding Sites on gp120 and Reverses T Cell Exhaustion via Impeding Tim-3 and PD-1 Upregulation - PubMed (original) (raw)
A Cinnamon-Derived Procyanidin Compound Displays Anti-HIV-1 Activity by Blocking Heparan Sulfate- and Co-Receptor- Binding Sites on gp120 and Reverses T Cell Exhaustion via Impeding Tim-3 and PD-1 Upregulation
Bridgette Janine Connell et al. PLoS One. 2016.
Abstract
Amongst the many strategies aiming at inhibiting HIV-1 infection, blocking viral entry has been recently recognized as a very promising approach. Using diverse in vitro models and a broad range of HIV-1 primary patient isolates, we report here that IND02, a type A procyanidin polyphenol extracted from cinnamon, that features trimeric and pentameric forms displays an anti-HIV-1 activity against CXCR4 and CCR5 viruses with 1-7 μM ED50 for the trimer. Competition experiments, using a surface plasmon resonance-based binding assay, revealed that IND02 inhibited envelope binding to CD4 and heparan sulphate (HS) as well as to an antibody (mAb 17b) directed against the gp120 co-receptor binding site with an IC50 in the low μM range. IND02 has thus the remarkable property of simultaneously blocking gp120 binding to its major host cell surface counterparts. Additionally, the IND02-trimer impeded up-regulation of the inhibitory receptors Tim-3 and PD-1 on CD4+ and CD8+ cells, thereby demonstrating its beneficial effect by limiting T cell exhaustion. Among naturally derived products significantly inhibiting HIV-1, the IND02-trimer is the first component demonstrating an entry inhibition property through binding to the viral envelope glycoprotein. These data suggest that cinnamon, a widely consumed spice, could represent a novel and promising candidate for a cost-effective, natural entry inhibitor for HIV-1 which can also down-modulate T cell exhaustion markers Tim-3 and PD-1.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: Drs Ekambaranellore Prakash and Viswaraman Mohan are employees of Indus Biotech, which provided the IND-02 compound and supported this work. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
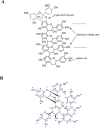
Fig 1. Structure of IND02.
Structure of the IND02 compounds, isolated from cinnamon and characterized as pentameric (A) or trimeric (B) procyanidin A (see HPLC analyses in Figures A and B in S1 File).
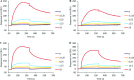
Fig 2. IND02 and IND02-trimer binding to gp120 immobilized sensorchip.
IND02 (A and B) and IND02-trimer (C and D) at the indicated concentrations (in μM) was injected at 25 μl/min for 5 minutes over the immobilized gp120, and the specific binding response in resonance unit (RU) was recorded as a function of time (S). Overlay of the sensorgrams is depicted for IND02 and IND02-trimer over MN- (A and C) and YU2- gp120 (B and D) respectively. After each injection, the surface was regenerated by a short pulse of 10 mM NaOH.
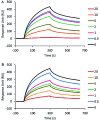
Fig 3. IND02 and IND02-trimer inhibition of gp120 binding to immobilized HS.
HIV-1 gp120 (MN) was injected over the immobilized HS surface at 10 μl/min in the presence or absence of IND02 (A) or IND02-trimer (B) at the indicated concentrations (in μM). The specific binding responses in resonance unit (RU) was recorded as a function of time (s), and after each injection the surface was regenerated by a short pulse of 0.05% SDS and 2M NaCl.
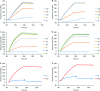
Fig 4. IND02 and IND02-trimer inhibition of gp120 binding to immobilized CD4 and mAb 17b.
HIV-1 gp120 was injected over the immobilized CD4 at 10 μl/min in the presence or absence of IND02 at the indicated concentrations (in μM). The specific binding responses in resonance unit (RU) for MN- (A) and YU2- (B) gp120 were recorded as a function of time (s). Similarly gp120/CD4 complex (made by pre incubating gp120 and CD4 both at 25 mM for 30 minutes at room temperature) was injected over the immobilized mAb 17b surfaces at 10 μl/min in the presence or absence of IND02 (C,D) or IND02-trimer (E,F) at the indicated concentrations (in μM). Incubations with the inhibitor lasted at least 1 hour. The specific binding responses in resonance unit (RU) for MN- (C, E) and YU2- (D, F) gp120 were recorded as a function of time (s), and after each injection, the surface was regenerated by a short pulse of 10 mM HCl.
Fig 5
Reduction of Tim-3 (left) and PD1 (right) expression on HIV-1 and IND02-trimer-treated CD8 + (A) and CD4 + (B) T cells. Stimulated PBMCs were simultaneously incubated with 0.4 to 40 μg/ml (0.46–46.3 μM) IND02-trimer and HIV-1 (92UG037, BaL, YU2b, 93BR020 or NL4-3). Up-regulation of Tim-3 (left) and PD-1 (right) on CD8+ (A) and CD4+ (B) T cells was impeded by the IND02-trimer, as shown by multicolour FACS analyses of live T cells on day 9 post co-treatment. Experiments were repeated in 4–5 independent experiments with cells from different donors and statistical differences were analyzed by the GraphPad prism software using the unpaired student’s t-test (2-tailed).
Similar articles
- A cinnamon-derived procyanidin type A compound inhibits hepatitis C virus cell entry.
Fauvelle C, Lambotin M, Heydmann L, Prakash E, Bhaskaran S, Vishwaraman M, Baumert TF, Moog C. Fauvelle C, et al. Hepatol Int. 2017 Sep;11(5):440-445. doi: 10.1007/s12072-017-9809-y. Epub 2017 Jul 11. Hepatol Int. 2017. PMID: 28698985 - Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds.
Madani N, Princiotto AM, Zhao C, Jahanbakhshsefidi F, Mertens M, Herschhorn A, Melillo B, Smith AB 3rd, Sodroski J. Madani N, et al. J Virol. 2017 Jan 18;91(3):e01880-16. doi: 10.1128/JVI.01880-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881646 Free PMC article. - Expression of PD-1 and Tim-3 markers of T-cell exhaustion is associated with CD4 dynamics during the course of untreated and treated HIV infection.
Rallón N, García M, García-Samaniego J, Cabello A, Álvarez B, Restrepo C, Nistal S, Górgolas M, Benito JM. Rallón N, et al. PLoS One. 2018 Mar 8;13(3):e0193829. doi: 10.1371/journal.pone.0193829. eCollection 2018. PLoS One. 2018. PMID: 29518102 Free PMC article. - A novel class of HIV-1 inhibitors that targets the viral envelope and inhibits CD4 receptor binding.
Wang HG, Williams RE, Lin PF. Wang HG, et al. Curr Pharm Des. 2004;10(15):1785-93. doi: 10.2174/1381612043384565. Curr Pharm Des. 2004. PMID: 15180540 Review. - Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition.
Connell BJ, Lortat-Jacob H. Connell BJ, et al. Front Immunol. 2013 Nov 20;4:385. doi: 10.3389/fimmu.2013.00385. Front Immunol. 2013. PMID: 24312095 Free PMC article. Review.
Cited by
- Litchi procyanidins inhibit colon cancer proliferation and metastasis by triggering gut-lung axis immunotherapy.
Yao Y, Feng S, Li X, Liu T, Ye S, Ma L, Man S. Yao Y, et al. Cell Death Dis. 2023 Feb 11;14(2):109. doi: 10.1038/s41419-022-05482-5. Cell Death Dis. 2023. PMID: 36774343 Free PMC article. - Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins.
Maffei ME, Salata C, Gribaudo G. Maffei ME, et al. Molecules. 2022 Nov 30;27(23):8353. doi: 10.3390/molecules27238353. Molecules. 2022. PMID: 36500445 Free PMC article. Review. - Curcumin-Loaded Silica Nanoparticles: Applications in Infectious Disease and Food Industry.
Maleki Dizaj S, Sharifi S, Tavakoli F, Hussain Y, Forouhandeh H, Hosseiniyan Khatibi SM, Memar MY, Yekani M, Khan H, Goh KW, Ming LC. Maleki Dizaj S, et al. Nanomaterials (Basel). 2022 Aug 18;12(16):2848. doi: 10.3390/nano12162848. Nanomaterials (Basel). 2022. PMID: 36014710 Free PMC article. Review. - Procyanidin C1 Location, Interaction, and Aggregation in Two Complex Biomembranes.
Villalaín J. Villalaín J. Membranes (Basel). 2022 Jul 5;12(7):692. doi: 10.3390/membranes12070692. Membranes (Basel). 2022. PMID: 35877895 Free PMC article. - Procyanidins: From Agro-Industrial Waste to Food as Bioactive Molecules.
Valencia-Hernandez LJ, Wong-Paz JE, Ascacio-Valdés JA, Chávez-González ML, Contreras-Esquivel JC, Aguilar CN. Valencia-Hernandez LJ, et al. Foods. 2021 Dec 20;10(12):3152. doi: 10.3390/foods10123152. Foods. 2021. PMID: 34945704 Free PMC article. Review.
References
- Jung M, Lee S, Kim H. Recent studies on natural products as anti-HIV agents. Curr Med Chem. 2000;7(6):649–61. . - PubMed
- Matthee G, Wright AD, Konig GM. HIV reverse transcriptase inhibitors of natural origin. Planta Med. 1999;65(6):493–506. . - PubMed
- Pal Singh I, Bharate SB, Bhutani KK. Anti-HIV natural products. Current Science. 2005;89(2):269–90.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials