Glycan-protein interactions in viral pathogenesis - PubMed (original) (raw)
Review
Glycan-protein interactions in viral pathogenesis
Rahul Raman et al. Curr Opin Struct Biol. 2016 Oct.
Abstract
The surfaces of host cells and viruses are decorated by complex glycans, which play multifaceted roles in the dynamic interplay between the virus and the host including viral entry into host cell, modulation of proteolytic cleavage of viral proteins, recognition and neutralization of virus by host immune system. These roles are mediated by specific multivalent interactions of glycans with their cognate proteins (generally termed as glycan-binding proteins or GBPs or lectins). The advances in tools and technologies to chemically synthesize and structurally characterize glycans and glycan-GBP interactions have offered several insights into the role of glycan-GBP interactions in viral pathogenesis and have presented opportunities to target these interactions for novel antiviral therapeutic or vaccine strategies. This review covers aspects of role of host cell surface glycan receptors and viral surface glycans in viral pathogenesis and offers perspectives on how to employ various analytical tools to target glycan-GBP interactions.
Copyright © 2016. Published by Elsevier Ltd.
Figures
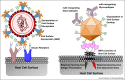
Figure 1
Schematic of complex glycans in the interplay between virus and host. Shown in the figure (on the left) is a schematic of a virus surface glycoprotein (such as influenza A virus hemagglutinin) that recognized glycans on the host cell surface as their primary receptors for viral attachment and entry. The viral surface protein is in itself glycosylated and depending on the site of glycan occupancy, the glycosylation would impact the binding of this protein to the host-cell glycan receptor. Shown on the right is a schematic of glycan on the surface of virus envelop proteins (such as dengue) interacting with GBP anchored on the host cell. This interaction could either be beneficial for the virus wherein it plays a role in viral attachment and entry into a cell capable of promoting the productive infection or it could be beneficial to the host wherein antigen presenting cells could uptake the virus and prime the host immune response.
Figure 2
Tools to define glycan-specific targets in viral–host interactions. (a) Shows snapshots of various analytical tools that either directly probe glycans in the appropriate physiological context (such as lectin-based staining and lectin array) or provide detailed characterization of glycans isolated from host or viral surface (using mass spectrometry and NMR based approaches. (b) Shows analysis of GBPs and their glycan-binding site using a network approach which not only takes into account key residues in glycan-binding but also those that are related to these residues through their inter-residue interaction network. (c) Shows a schematic of biochemical (top) and structural (bottom) tools to probe glycan–GBP interactions. As shown in the bottom, shape based definitions of the conformational space sampled by glycans relates the conformations defined by 7 different glyosidic torsion angle (for a tetrasaccharide) into a single parameter θ whose variation can be studied during molecular dynamics simulations.
Similar articles
- Structural features of glycan recognition among viral pathogens.
Shanker S, Hu L, Ramani S, Atmar RL, Estes MK, Venkataram Prasad BV. Shanker S, et al. Curr Opin Struct Biol. 2017 Jun;44:211-218. doi: 10.1016/j.sbi.2017.05.007. Epub 2017 Jun 4. Curr Opin Struct Biol. 2017. PMID: 28591681 Free PMC article. Review. - The Importance of Glycans of Viral and Host Proteins in Enveloped Virus Infection.
Li Y, Liu D, Wang Y, Su W, Liu G, Dong W. Li Y, et al. Front Immunol. 2021 Apr 29;12:638573. doi: 10.3389/fimmu.2021.638573. eCollection 2021. Front Immunol. 2021. PMID: 33995356 Free PMC article. Review. - Initial Step of Virus Entry: Virion Binding to Cell-Surface Glycans.
Koehler M, Delguste M, Sieben C, Gillet L, Alsteens D. Koehler M, et al. Annu Rev Virol. 2020 Sep 29;7(1):143-165. doi: 10.1146/annurev-virology-122019-070025. Epub 2020 May 12. Annu Rev Virol. 2020. PMID: 32396772 Review. - Novel Functions of Hendra Virus G N-Glycans and Comparisons to Nipah Virus.
Bradel-Tretheway BG, Liu Q, Stone JA, McInally S, Aguilar HC. Bradel-Tretheway BG, et al. J Virol. 2015 Jul;89(14):7235-47. doi: 10.1128/JVI.00773-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948743 Free PMC article. - Fluorescence-based solid-phase assays to study glycan-binding protein interactions with glycoconjugates.
Leppänen A, Cummings RD. Leppänen A, et al. Methods Enzymol. 2010;478:241-64. doi: 10.1016/S0076-6879(10)78012-5. Methods Enzymol. 2010. PMID: 20816484
Cited by
- Lactoferrin-Conjugated Nanoparticles as New Antivirals.
Krzyzowska M, Janicka M, Tomaszewska E, Ranoszek-Soliwoda K, Celichowski G, Grobelny J, Szymanski P. Krzyzowska M, et al. Pharmaceutics. 2022 Sep 3;14(9):1862. doi: 10.3390/pharmaceutics14091862. Pharmaceutics. 2022. PMID: 36145610 Free PMC article. Review. - N-Linked Glycosylation on Anthrax Toxin Receptor 1 Is Essential for Seneca Valley Virus Infection.
Jayawardena N, Miles LA, Burga LN, Rudin C, Wolf M, Poirier JT, Bostina M. Jayawardena N, et al. Viruses. 2021 Apr 28;13(5):769. doi: 10.3390/v13050769. Viruses. 2021. PMID: 33924774 Free PMC article. - The Influence of Viral Infections on Iron Homeostasis and the Potential for Lactoferrin as a Therapeutic in the Age of the SARS-CoV-2 Pandemic.
Ward JL, Torres-Gonzalez M, Ammons MCB. Ward JL, et al. Nutrients. 2022 Jul 27;14(15):3090. doi: 10.3390/nu14153090. Nutrients. 2022. PMID: 35956266 Free PMC article. Review. - Comparative Assessment of Water Models in Protein-Glycan Interaction: Insights from Alchemical Free Energy Calculations and Molecular Dynamics Simulations.
Li D, Minkara MS. Li D, et al. J Chem Inf Model. 2024 Dec 23;64(24):9459-9473. doi: 10.1021/acs.jcim.4c01361. Epub 2024 Oct 8. J Chem Inf Model. 2024. PMID: 39378441 Free PMC article. - Double-Modified Glycopolymers from Thiolactones to Modulate Lectin Selectivity and Affinity.
Wilkins LE, Badi N, Du Prez F, Gibson MI. Wilkins LE, et al. ACS Macro Lett. 2018 Dec 18;7(12):1498-1502. doi: 10.1021/acsmacrolett.8b00825. Epub 2018 Dec 6. ACS Macro Lett. 2018. PMID: 30662815 Free PMC article.
References
- Van Breedam W., Pohlmann S., Favoreel H.W., de Groot R.J., Nauwynck H.J. Bitter-sweet symphony: glycan–lectin interactions in virus biology. FEMS Microbiol Rev. 2014;38:598–632. - PMC - PubMed
- This is a review of outstanding interest given that it captures all the known mammalian host lectins known to play key roles in viral pathogensis through their interactions with viral surface glycosylation.
- Stroh L.J., Stehle T. Glycan engagement by viruses: receptor switches and specificity. Annu Rev Virol. 2014;1:285–306. - PubMed
- This is a review of special interest given that it captures knowledge on virus–host systems where specificity of interaction with glycan receptor plays a key role in host tropism and viral pathogenesis.
- Varki A., Cummings R.D., Esko J.D., Freeze H.H., Hart G.W., Etzler M.E. edn 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. Essentials of glycobiology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials